Product Number: C062010
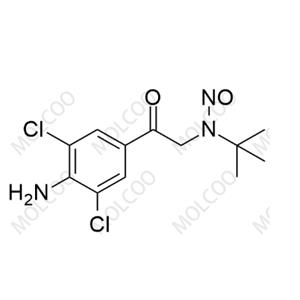
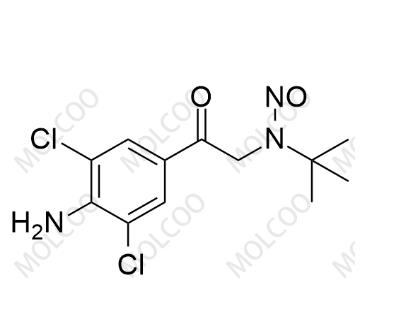
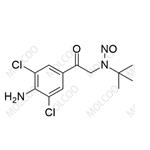
English Name: N-Nitroso Clenbuterol Impurity 2
English Alias: N-(2-(4-amino-3,5-dichlorophenyl)-2-oxoethyl)-N-(tert-butyl)nitrous amide
CAS Number: None
Molecular Formula: C12H15Cl2N3O2
Molecular Weight: 304.17
Advantages: N-Nitroso Clenbuterol Impurity 2 is synthesized using cutting-edge technology and refined purification processes, boasting extremely high purity and excellent stability. Through advanced analytical instruments and strict quality inspection systems, its accurate chemical structure and highly uniform composition are ensured. It can provide a precise and reliable reference standard for drug research, development, and quality testing, effectively reducing experimental errors and enhancing the accuracy and reliability of data.
Applications: It is mainly applied to the fields of impurity research, quality analysis, and control of Clenbuterol drugs. During the drug research and development process, it can be used to determine the content of this impurity in drugs and evaluate its potential impact on drug safety and effectiveness. In the quality control of pharmaceutical production, as a key reference substance, it can accurately detect the presence of impurities in products, helping enterprises strictly control drug quality and ensure compliance with relevant domestic and international regulations and quality standards. It also plays an important role in drug safety evaluation and other research.
Background Description: With the continuous upgrading of requirements for drug quality and safety in the pharmaceutical industry, the research on drug impurities is a crucial part of ensuring drug quality. Although Clenbuterol has received much attention due to illegal use, in legal medical applications, the control of its impurities is related to medication safety and treatment effects. N-Nitroso Clenbuterol Impurity 2, as one of the potential impurities in the drug, in-depth research on it helps to improve the drug quality control system, ensure patient medication safety, and enhance the overall quality of drugs.
Research Status: Currently, research on N-Nitroso Clenbuterol Impurity 2 is gradually deepening. Researchers are actively developing more sensitive and efficient detection technologies, such as ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), to achieve trace detection and precise quantification of this impurity. Meanwhile, studies on its generation mechanism during drug synthesis, its interaction with drug active ingredients, as well as its impact on drug stability and efficacy, are also actively carried out, aiming to provide more comprehensive theoretical support and technical guidance for the research, development, production, and quality control of Clenbuterol drugs.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!









 China
China