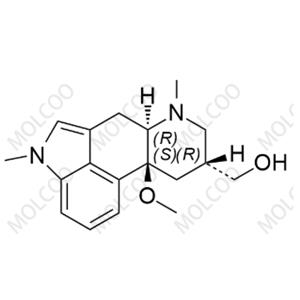
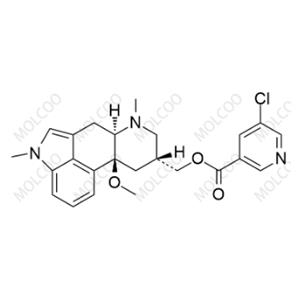
Product Details
| Product Name: Nicergoline Impurity C | CAS No.: 35155-28-3 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/07/31 |
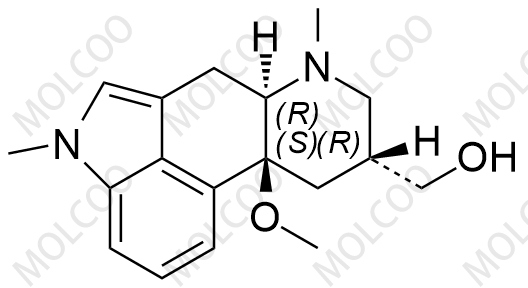
Nicergoline Impurity Reference Standards
Nicergoline Impurity Reference Standards are crucial substances for drug research and development, quality control, and impurity analysis. These reference standards ensure the purity and quality of Nicergoline medications during research and production processes, and they also aid in identifying and quantifying potential impurities in the drug.
Product Characteristics
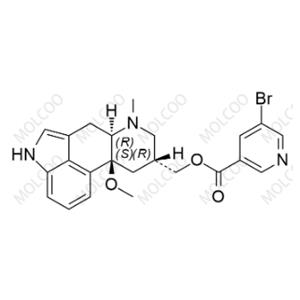
Rich Variety of Impurities: Covering a wide range of Nicergoline impurities such as A, B, C, D, E, F, G, H, I, and J, to meet diverse research and production needs.
High Purity: Rigorous synthesis and purification processes ensure that the purity of the impurity reference standards exceeds 95%.
Excellent Stability: Adopting appropriate storage conditions to ensure the stability of the impurity reference standards over time.
Application Scope
Drug Research and Development: During the drug research and development stage, Nicergoline Impurity Reference Standards can be used to establish impurity profiles, helping researchers understand the types and concentrations of impurities that may be present in the drug.
Quality Control: In production processes, these reference standards can be used for quality control and impurity detection, ensuring each batch of the drug meets established quality standards.
Impurity Analysis: In drug analysis laboratories, Nicergoline Impurity Reference Standards can be used for qualitative and quantitative impurity analysis, providing strong support for drug quality control.
Product Advantages
Professional Production: We have a professional production team and advanced production equipment to ensure the quality and stability of the impurity reference standards.
Excellent Service: We provide comprehensive technical support and after-sales service, including product consultation, usage guidance, etc., to ensure that users receive timely assistance and guidance during use.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $/ |
VIP5Y
|
RongNa Biotechnology Co.,Ltd
|
2025-04-29 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-04-22 | |
| $44.00/25mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-11-10 |






 China
China