
Product Details
| Product Name: Vismodegib Impurity | CAS No.: 94088-68-3 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C14H14ClNO7 |
Vismodegib Impurity;94088-68-3
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: V043006
English Name: Vismodegib Impurity 6
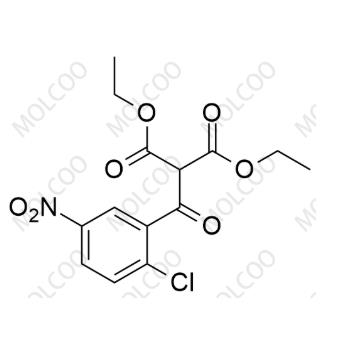
English Alias: diethyl 2-(2-chloro-5-nitrobenzoyl)malonate
CAS Number: 94088-68-3
Molecular Formula: C₁₄H₁₄ClNO₇
Molecular Weight: 343.72
Advantages
Well-defined and highly characteristic structure: Containing chlorine atom, nitro group, benzoyl group, and diethyl malonate structure, it is significantly different from the benzamide structure of Vismodegib. It can be accurately identified by techniques like HPLC and GC-MS, providing a specific marker for impurity detection;
High stability and traceability: The ester and nitro structures are stable under conventional storage conditions. As a potential intermediate in the acylation step of Vismodegib synthesis, it can directly reflect incomplete condensation between diethyl malonate and nitrobenzoyl chloride, improving the accuracy of process tracing;
High detection sensitivity: The conjugated structure of nitro and benzoyl groups has strong absorption in the UV region, enabling trace analysis via HPLC-UV, reducing detection costs and enhancing method applicability.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Impurity 6 in Vismodegib APIs, ensuring residual acylation intermediates meet quality standards during synthesis;
Synthesis process optimization: Reducing intermediate residues and improving main product yield by monitoring the impurity content and optimizing condensation catalysts (e.g., triethylamine) and reaction temperature;
Process validation: Used to verify the stability of the acylation step in Vismodegib synthesis, ensuring consistency of impurity content across batches.
Background Description
Research Status
Detection method optimization: Using HPLC-DAD with optimized separation conditions based on the characteristic UV absorption (254nm) of nitro and benzoyl groups to achieve trace detection (detection limits up to ppm level);
Synthesis process improvement: Enhancing condensation conversion by adjusting solvents (e.g., dichloromethane) and raw material ratios to reduce intermediate residues;
Impurity conversion studies: Exploring the transformation pathway of this impurity in subsequent decarboxylation and nitro reduction steps to assess its impact on final product purity;
Quality standard development: Establishing reasonable impurity limits based on multi-batch production data, integrating them into the quality control system for Vismodegib APIs
Product Information
Product Number: V043006
English Name: Vismodegib Impurity 6
English Alias: diethyl 2-(2-chloro-5-nitrobenzoyl)malonate
CAS Number: 94088-68-3
Molecular Formula: C₁₄H₁₄ClNO₇
Molecular Weight: 343.72
Advantages
Well-defined and highly characteristic structure: Containing chlorine atom, nitro group, benzoyl group, and diethyl malonate structure, it is significantly different from the benzamide structure of Vismodegib. It can be accurately identified by techniques like HPLC and GC-MS, providing a specific marker for impurity detection;
High stability and traceability: The ester and nitro structures are stable under conventional storage conditions. As a potential intermediate in the acylation step of Vismodegib synthesis, it can directly reflect incomplete condensation between diethyl malonate and nitrobenzoyl chloride, improving the accuracy of process tracing;
High detection sensitivity: The conjugated structure of nitro and benzoyl groups has strong absorption in the UV region, enabling trace analysis via HPLC-UV, reducing detection costs and enhancing method applicability.
Applications
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Impurity 6 in Vismodegib APIs, ensuring residual acylation intermediates meet quality standards during synthesis;
Synthesis process optimization: Reducing intermediate residues and improving main product yield by monitoring the impurity content and optimizing condensation catalysts (e.g., triethylamine) and reaction temperature;
Process validation: Used to verify the stability of the acylation step in Vismodegib synthesis, ensuring consistency of impurity content across batches.
Background Description
Research Status
Detection method optimization: Using HPLC-DAD with optimized separation conditions based on the characteristic UV absorption (254nm) of nitro and benzoyl groups to achieve trace detection (detection limits up to ppm level);
Synthesis process improvement: Enhancing condensation conversion by adjusting solvents (e.g., dichloromethane) and raw material ratios to reduce intermediate residues;
Impurity conversion studies: Exploring the transformation pathway of this impurity in subsequent decarboxylation and nitro reduction steps to assess its impact on final product purity;
Quality standard development: Establishing reasonable impurity limits based on multi-batch production data, integrating them into the quality control system for Vismodegib APIs
Applications
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1g |
VIP2Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-07-24 | |
| $0.00/1kg |
VIP1Y
|
Jinan Ruitong Biotech Co., Ltd.
|
2025-08-22 | |
| $38.00/5mg |
VIP6Y
|
TargetMol Chemicals Inc.
|
2025-11-09 |








 China
China