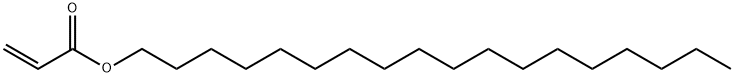
Application research of Octadecyl acrylate
Nov 18,2025
Introduction
Octadecyl acrylate (Figure 1) is an important organic chemical raw material. Its monomer, homopolymer or copolymer with styrene, maleic anhydride (or ester) and other monomers are widely used as plasticizer, adhesive, fiber treatment agent, paper finishing agent, product oil flow modifier and pour point depressant of crude oil.

Octadecyl acrylate Preparations
The factors effecting the melt direct esterification and normal solvent direct esterification of acrylic acid and octadecyl alcohol were investigated. The optimum preparation conditions for melt direct esterification were: molar of acrylic acid to octadecyl alcohol 1.2, 3.0% toluene-p-sulfonic acid as catalyst, 1.0% hydroqninone as inhibitor and reflux at 110~120℃ for 8h and the yield was more than 90%. The optimum preparation conditions for solvent direct esterification were: molar ratio of acrylic acid to octadecyl alcohol 1.2:1.0%, toluene-p-sulfonic acid 1.0%, 0.5% hydroqninone and reacting 6h, and the yield was also 90% or more. The composition, properties and structure of octadecyl acrylate from different preparation methods were identified and evaluated by melting point and boiling point test elementary analysis, IR spectroscopy and 1H-NMR spectroscopy. The experimental results showed that the two octadecyl acrylate products are similar in physical and structural characteristics. The melt direct esterification is a more efficient and reliable preparation method for octadecyl acrylate because of the smaller amount of catalyst and inhibitor used and the higher yield.[1]
Poly(Octadecyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels
Stimuli-sensitive hydrogels are highly desirable candidates for application in intelligent biomaterials. Thus, a novel thermosensitive hydrogel with shape-memory function was developed. Hydrophobic octadecyl acrylate (OA), hydrophilic methoxy poly(ethylene glycol) acrylate (MPGA), and a crosslinking monomer were copolymerized to prepare poly(OA-co-MPGA) gels with various mole fractions of OA (xOA) in ethanol. Subsequently, the prepared gels were washed, dried, and re-swelled in water at 50°C. Differential scanning calorimetric (DSC) and compression tests at different temperatures revealed that poly(OA-co-MPGA) hydrogels with xOA>0.5 induce a crystalline-to-amorphous transition, which is a hard-to-soft transition at ~40°C that is based on the formation/non-formation of a crystalline structure containing stearyl side chains. The hydrogels stored in water maintained an almost constant volume, independent of the temperature. The poly(OA-co-MPGA) hydrogel was soft, flexible, and deformed at 50°C. However, the hydrogel stiffened when cooled to room temperature, and the deformation was reversible. The shape-memory function of poly(OA-co-MPGA) hydrogels is proposed for potential use in biomaterials; this is partially attributed to the use of MPGA, which consists of relatively biocompatible poly(ethylene glycol).[2]
Poly(perfluoroalkyl acrylate-co-Octadecyl acrylate)
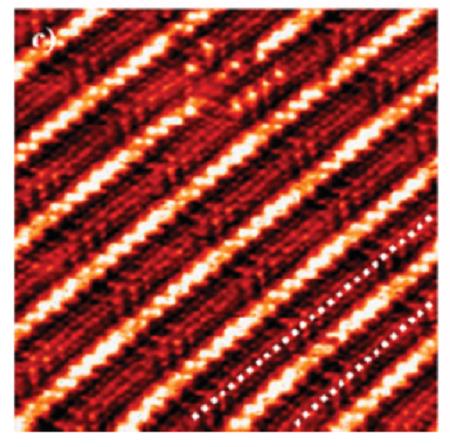
Novel fluorinated copolymers of Octadecyl acrylate (OA) and (perfluorohexyl)ethyl acrylate (C6A), (perfluorohexyl)ethyl methacrylate (C6MA), 2-[[[[2-(perfluorohexyl)]-sulfonyl]methyl] amino]ethyl acrylate (C6SA), and methacrylate (C6SMA) were synthesized via miniemulsion copolymerization. The extremely hydrophobic monomers perfluoroalkyl acrylate (FA) and octadecyl acrylate acted as the reactive costabilizer in the miniemulsion system. The microstructure and surface wetting properties of the copolymers were characterized by 1H-NMR, FT-IR, and dynamic contact angle test. The crystallization behaviors and fine surface structures of the copolymer films were determined by differential scanning calorimetry (DSC) and wide-angle X-ray diffraction (WAXD) analysis. The self-assembled aggregation and roughness of the copolymer films were investigated by atomic force microscopy (AFM). The results showed that the fluorinated side chains interrupted and impeded the crystallizable side chains of octadecyl acrylate from forming complete crystals. And the Tm and ΔHf of the copolymers were decreased as a consequence of this effect. The fluorinated side chains in P(C6A/OA) and P(C6MA/OA) arranged between the crystallizable hydrocarbon side chains of octadecyl acrylate, while the crystallization structure of fluorinated and nonfluorinated pendant groups existed all at once in copolymers P(C6SA/OA) and P(C6SMA/OA). The four copolymers exhibited very low surface free energy and excellent dynamic water repellency attributed to the restriction of perfluoroalkyl groups combined with crystallization of stearyl pendant groups.[3]
Polymer Nanoemulsion
The demand for waterproof leather has been increasing, and environmentally friendly waterproof fatliquors have recently received increasing attention. In this work, two polymer nanoemulsions containing carboxyl groups were synthesized and used as waterproof fatliquors for chrome-tanned leather. First, a reactive emulsifier (C12-Na) was prepared using itaconic anhydride and lauryl alcohol. Subsequently, two polymer nanoemulsions were prepared through mini-emulsion polymerization with C12-Na as the emulsifier, 4,4'-azobis (4-cyanovaleric acid) as the initiator, and lauryl acrylate (LA)/Octadecyl acrylate (OA) as monomers; these were named PLA and POA. PLA and POA were characterized using FT-IR, a Zetasizer, and GPC. It was found that the critical micellar concentration (CMC) of C12-Na was 2.34 mmol/L, which could reduce the surface tension of water to 26.61 mN/m. The average particle sizes of PLA and POA were 53.39 and 67.90 nm, respectively. The maeser flexes of leather treated with PLA and POA were 13928 and 19492 at a 5% dosage, respectively, and the contact angles reached 148.4° and 150.3°, respectively; these values were both higher than for a conventional fatliquor. Compared with PLA, the leather treated with POA exhibited better fullness, and tensile and tearing strength. The prepared nanoemulsions have prospective applications in leather manufacturing as waterproof fatliquors.[4]
Poly(N-isopropylacrylamide-co-Octadecyl acrylate)
Copolymers of N-isopropylacrylamide and Octadecyl acrylate (PNIPA-OAX) with various Octadecyl acrylate feed ratios (X = 1-10 mol%) were synthesized and electrospun into nanofiber mats. It was found that average diameter of nanofibers electrospun at concentration of 25% and voltage of 30 kV linearly increased from 165 nm (PNIPA) to 497 nm (PNIPA-OA10) with increasing the octadecyl acrylate content. The PNIPA-OAX (X=3-10 mol%) nanofiber mats were insoluble in water at 25 degrees C, in which inter-polymer and interfiber physical cross-links were formed through hydrophobic interaction of stearyl side-chains. With increasing the temperature from 25 to 40 degrees C the PNIPA-OA3 nanofiber mat exhibited significant volume contraction of 66%, while that of a single nanofiber estimated by AFM measurements was found to be 37%. The results allowed us to conclude that not only swelling-deswelling but also dissociation-association of the nanofibers via hydrophobic interactions were crucially important for the macroscopic volume changes of the nanofiber mats.[5]
References
1. Zhou FS etal,.Evaluation on the Preparations of Octadecyl Acrylate[J].Chemical World,2002,(03):143-146.DOI:10.19500/j.cnki.0367-6358.2002.03.010.
2. Tokuyama H, Iriki R, Kubota M. Thermosensitive Shape-Memory Poly(stearyl acrylate-co-methoxy poly(ethylene glycol) acrylate) Hydrogels. Gels. 2023;9(1):54. Published 2023 Jan 10. doi:10.3390/gels9010054
3. Zhang Q, Wang Q, Jiang J, Zhan X, Chen F. Microphase Structure, Crystallization Behavior, and Wettability Properties of Novel Fluorinated Copolymers Poly(perfluoroalkyl acrylate-co-stearyl acrylate) Containing Short Perfluorohexyl Chains. Langmuir. 2015;31(16):4752-4760. doi:10.1021/la504467m
4. Jin L, Xu W, Wen H, Wang Y, Zhang F. Imparting Waterproofing Properties to Leather by Polymer Nanoemulsion Based on Long-Chain Alkyl Acrylate. Materials (Basel). 2023;16(4):1464. Published 2023 Feb 9. doi:10.3390/ma16041464
5. Okuzaki H, Kobayashi K, Hishiki F, Su SJ, Yan H. Thermo-responsive nanofiber mats fabricated by electrospinning of poly(N-isopropylacrylamide-co-stearyl acrylate). J Nanosci Nanotechnol. 2011;11(6):5193-5198. doi:10.1166/jnn.2011.4186
- Related articles
- Related Qustion
A review of 1-bromoheptane’s surface adsorption structures, self-assembly behavior, photodissociation dynamics, and glutathione-related biochemical effects.....
Nov 18,2025Organic Chemistry4,4'-Dibromobiphenyl is the most typical representative of brominated flame retardant and is also an important intermediate. Its application were reported.....
Nov 18,2025Organic Synthesis IntermediateOctadecyl acrylate
4813-57-4You may like
Octadecyl acrylate manufacturers
- Octadecyl acrylate
-

- $10.00 / 1KG
- 2025-12-11
- CAS:4813-57-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5tons
- Stearyl Acrylate
-

- $0.00 / 200kg
- 2025-11-19
- CAS:4813-57-4
- Min. Order: 20kg
- Purity: 96.0%
- Supply Ability: 10 tons
- Octadecyl acrylate
-
- $100.00 / 1KG
- 2025-09-25
- CAS:4813-57-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available