Prilocaine Hydrochloride--Metabolism,Excretion,Pediatric use, Biological Functions
Oct 12,2020
Classification. Amide.
Other chemical (generic) name. Propitocaine (Japan).
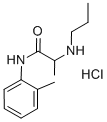
Chemical formula. 2-Propylamino-o-propionotoluidide hydrochloride.
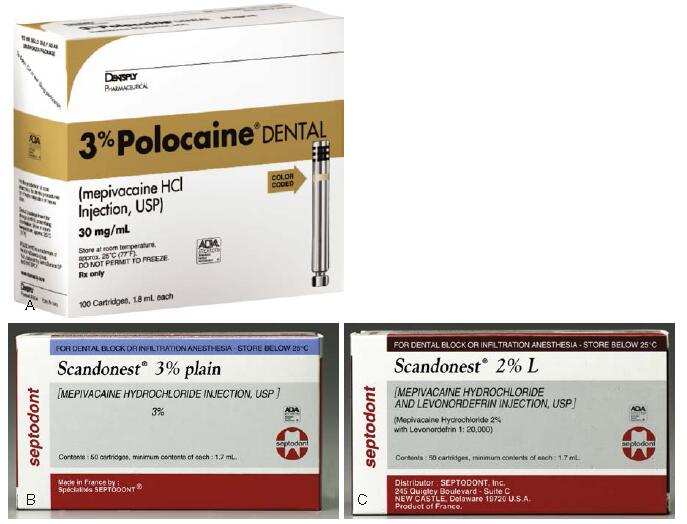
Fig. 1 (A and B) Mepivacaine, 3%. (C) Mepivacaine, 2%, with levonordefrin 1:20,000. ([A] Courtesy Dentsply, York, Pennsylvania, United States, [B and C] Courtesy of Septodont, Inc., Lancaster, PA.)
Prepared by. Löfgren and Tegnér, 1953; reported in 1960.
FDA approved. November 1965.
Potency. 2 (procaine = 1; lidocaine = 2).
Toxicity. 1 (procaine = 1; lidocaine = 2); 40% less toxic than lidocaine.
Metabolism. The metabolism of prilocaine differs significantly from that of lidocaine and mepivacaine. Because it is a secondary amine, prilocaine is hydrolyzed straight forwardly by hepatic amidases to o-toluidine and N-propylalanine. Carbon dioxide is a major end product of prilocaine biotransformation. The efficiency of the body’s degradation of prilocaine is demonstrated by the extremely small fraction of intact prilocaine recoverable in the urine. o-Toluidine can induce the formation of methemoglobin, producing methemoglobinemia if large doses are administered.
Minor degrees of methemoglobinemia have been observed following both benzocaine and lidocaine administration, 30,31 but prilocaine consistently reduces the blood’s oxygen-carrying capacity, at times sufficiently to cause observable cyanosis.32-34 Limiting the total prilocaine dose to 600 mg (FDA recommendation) avoids symptomatic cyanosis. Methemoglobin blood levels of less than 20% do not usually produce clinical signs or symptoms (which include grayish or slate-blue cyanosis of the lips, mucous membranes, and nail beds and [infrequently] respiratory and circulatory distress). Methemoglobinemia may be reversed within 15 minutes with administration of 1 to 2 mg/kg body weight of 1% methylene blue solution intravenously over a 5-minute period.Prilocaine undergoes biotransformation more rapidly and completely than lidocaine. This occurs not only in the liver but also to a lesser degree in the kidneys and lungs.35,36 Plasma levels of prilocaine decrease more rapidly than those of lidocaine. Prilocaine is thus considered to be less toxic systemically than comparably potent local anesthetic amides.
Signs of CNS toxicity after prilocaine administration in humans are briefer and less severe than after the same intravenous dose of lidocaine. Excretion. Prilocaine and its metabolites are excreted primarily via the kidneys. Renal clearance of prilocaine is faster than for other amides, resulting in its faster removal from the circulation. Vasodilating properties. Prilocaine is a vasodilator. It produces greater vasodilation than is produced by mepivacaine but less than that produced by lidocaine and significantly less than that produced by procaine. pKa. 7.9.
pH of plain solution. Approximately 6.0 to 6.5.
pH of vasoconstrictor-containing solution. Approximately 4.0.
Onset of action. Slightly slower than that of lidocaine (3 to 5 minutes).
Effective dental concentration. 4%.
Anesthetic half-life. 1.6 hours.
Topical anesthetic action. Not in clinically acceptable concentrations. Prilocaine, in its uncharged base form, is an integral part of EMLA cream (eutectic mixture of the local anesthetics lidocaine and prilocaine), a formulation that permits anesthetics to penetrate the imposing anatomic barrier of intact skin. EMLA cream is used to provide topical anesthesia of skin before venipuncture and other painful cosmetic procedures. Oraqix, a noninjectable lidocaine-prilocaine gel, is used successfully to provide pain relief in association with periodontal probing and scaling/root planing when deposited in periodontal pockets, for rubber dam placement, gingival retraction, and placement of orthodontic temporary anchorage devices.
Pregnancy classification. B.
Nursing mothers. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when prilocaine is administered to a nursing woman.
Pediatric use. Doses in children should be reduced, commensurate with age, body weight, and physical condition.
Maximum recommended dose. The FDA MRD for prilocaine is 8.0 mg/kg (3.6 mg/lb) body weight for the adult patient, to an MRD of 600 mg.
Comments. The clinical actions of plain prilocaine (Fig. 4.4) vary significantly with the type of injection technique used. Although true for all anesthetics, the variation between supraperiosteal infiltration and nerve block is more pronounced with plain prilocaine (and plain mepivacaine). Infiltration provides short durations of pulpal (10 to 15 minutes) and soft tissue (1.5 to 2 hours) anesthesia, whereas regional nerve block (e.g., inferior alveolar nerve block) provides pulpal anesthesia for up to 60 minutes (commonly 40 to 60 minutes) and soft tissue anesthesia for 2 to 4 hours.41 Thus plain prilocaine is frequently able to provide anesthesia that is equal in duration—although not equal in depth—to that attained with lidocaine or mepivacaine with a vasoconstrictor.
The clinical actions of prilocaine with epinephrine 1:200,000 are not as dependent on anesthetic technique as with other dental local anesthetics. Prilocaine with epinephrine provides lengthy anesthesia while offering a less concentrated epinephrine solution: 1:200,000. Pulpal anesthesia of 60 to 90 minutes’ duration and soft tissue anesthesia of 3 to 8 hours’ duration are common. The cartridge contains 9 μg of epinephrine; therefore epinephrine-sensitive individuals, such as the ASA class 3 cardiovascular disease patient, may receive up to four cartridges (36 μg) of prilocaine with epinephrine.
Fig. 2 (A and B) Prilocaine, 4%. (C and D) Prilocaine, 4%, with epinephrine 1:200,000. (Courtesy Dentsply, York, Pennsylvania, United States.)
In epinephrine-sensitive patients requiring prolonged pulpal anesthesia (≥60 minutes), plain prilocaine or prilocaine with epinephrine 1:200,000 is strongly recommended. It is rapidly biotransformed and, for this reason, is considered to be a safe local anesthetic (e.g., lower toxicity).
Prilocaine is relatively contraindicated in patients with idiopathic or congenital methemoglobinemia, hemoglobinopathies (sickle cell anemia), anemia, or cardiac or respiratory failure evidenced by hypoxia because methemoglobin levels are increased, decreasing oxygen-carrying capacity.33,34,48 Prilocaine administration is also relatively contraindicated in patients receiving acetaminophen or phenacetin, both of which produce elevations in methemoglobin levels.
Prilocaine hydrochloride (Citanest) is an amide anesthetic whose onset of action is slightly longer than that of lidocaine; its duration of action is comparable. Prilocaine is 40% less toxic acutely than lidocaine, making it especially suitable for regional anesthetic techniques. It is metabolized by the liver to orthotoluidine, which when it accumulates, can cause conversion of hemoglobin (HB+++ to methemoglobin (HB+++. Oxygen transport is impaired in the presence of methemoglobinemia. Treatment involves the use of reducing agents, such as methylene blue, given intravenously, to reconvert methemoglobin to hemoglobin.
It has been claimed that 4% prilocaine hydrochloride solution (with or without a vasopressor) is associated with a higher risk of paresthesia, primarily of the lingual nerve, than other anesthetic formulations following its administration by inferior alveolar nerve block.
Although the “evidence” remains anecdotal, it appears that prilocaine, as formulated in North America (as a 4% solution), might be more neurotoxic than other commonly used local anesthetic formulations.
- Related articles
- Related Qustion
- Propitocaine hydrochloride: mechanism of action, clinical applications and side effects Nov 22, 2023
Propitocaine hydrochloride is a local anesthetic that blocks nerve impulses, providing temporary pain relief during various medical procedures.
Lidocaine hydrochloride is a local anesthetic and antiarrhythmic drug. It is clinically used for infiltration anesthesia, epidural anesthesia, surface anesthesia and nerve conduction block.....
Oct 10,2020AnestheticsArticaine hydrochloride with epinephrine is contraindicated in persons with known sensitivity to amide-type local anesthetics (few to none) and in persons with sulfite sensitivity (such as some asthmatic patients with allergic-type asthma,....
Oct 13,2020AnestheticsPropitocaine hydrochloride
1786-81-8You may like
- Lidocaine hydrochloride: A local anesthetic
Mar 19, 2025
- What is Atracurium Besylate Injection
Apr 15, 2024
- General Introduction of Caffeine
Mar 3, 2022
Propitocaine hydrochloride manufacturers
- Propitocaine hydrochloride
-

- $2.00 / 1kg
- 2025-12-17
- CAS:1786-81-8
- Min. Order: 0.10000000kg
- Purity: 99%
- Supply Ability: 200KG
- Propitocaine hydrochloride
-

- $5.00/ KG
- 2025-12-17
- CAS:1786-81-8
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
- Prilocaine HCl
-

- $0.00 / 1kg
- 2025-12-16
- CAS:1786-81-8
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 800kg