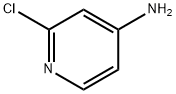
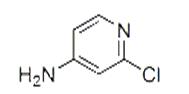
4-Amino-2-chloropyridine: Application, Synthesis
Dec 9,2019
4-Amino-2-chloropyridine is an important pharmaceutical and pesticide intermediate. It can synthesize N-(2-chloro-4-pyridyl)urea regulators that promote plant growth. It is also a key substance in the synthesis of KT-30 (Forchlorfenuron, IUPAC name N-(2-chloro-4-pyridyl)-N'-phenylurea). KT-30 is a highly active cytokinin, which has the biological activity of promoting tissue growth, promoting bud development and green preservation[1]; it has an effect of increasing yield on apple, pear, grape, peach and other fruit crops.
4-Amino-2-chloropyridine itself can also be used as a pesticide alone, and has high activity against various pathogens such as rust, powdery mildew, rice blast, and apple downy mildew. 4-Amino-2-chloropyridine and its pharmaceutical and pesticide intermediates have the advantages of high biological activity, low toxicity, long-lasting period and easy degradation in the environment, so they have broad application prospects in agricultural production.
There are many reports on the synthesis of 4-Amino-2-chloropyridine at home and abroad, such as 2-chloro-4-nitropyridine reduction method[2]; 2-chloro-4-cyanopyridine reduction method; Azide method; 2,4-dihydroxypyridine chlorination, amination[3]; Pyridine oxidation, nitration, rechlorination, ammoniation; 2-Aminopyridine first oxidation and then chlorination; 2-Aminopyridine first chlorination and then oxidation[4]. However, due to the difficulty in preparing raw materials and intermediates, which are highly toxic, flammable, explosive, and difficult to separate reaction products, most of the synthetic research is in the experimental stage, and it is difficult to achieve industrial amplification. Several industrially synthesized 4-Amino-2-chloropyridine methods are described below.
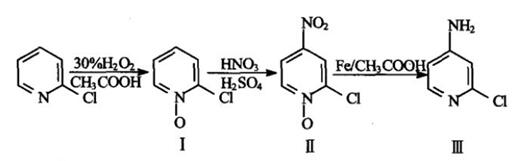
(1) The 2-Chloropyridine nitration reduction method is a common method for the synthesis of 4-Amino-2-chloropyridine[5]. The raw material 2-Chloropyridine is inexpensive, and the reagents required for the reaction are common chemical reagents, the steps are simple, and the reaction yield is high. A more economical synthetic route. 2-Chloropyridine in an acetic acid medium, heated to about 50 degrees Celsius, with 30% hydrogen peroxide to 2-Chloropyridine-N-oxide; the pyridine ring 4 carbon atoms are activated, so that the 4 carbon atoms are easy to introduce functional groups. However, due to the strong electron withdrawing group on the pyridine ring, the pyridine ring is passivated, which affects the final yield of the product; 2-Chloropyridine-N-oxide is nitrated in a sulfuric acid medium and introduced at the 4 carbon atom. The nitro group produces 2-chloro-4-nitropyridine-N-oxide; it is then reduced to give 4-Amino-2-chloropyridine. A common reducing agent is to add a certain amount of iron powder to acetic acid.
(2) Isonicotinic acid chlorination, ammoniation synthesis method[6]. Using isonicotinic acid as raw material, hydrogen peroxide is used to form isonicotinic acid N-oxide, and isocyanamide-N-oxide is obtained by amination. The pyridine ring position is activated, and nucleophilic substitution reaction is easy to occur. 2-chloro-4-isonicotinamide is formed by the action of phosphorus pentachloride, and finally 4-Amino-2-chloropyridine is formed by Hofmann degradation reaction under the action of alkaline sodium hypochlorite. The method uses cheaper isonicotinic acid as raw material, and the operation is simple, and the reaction is easy to control; however, the synthetic route is too long, and the raw material loss is excessive.
References
[1] Iwahori S , Tominaga S , Yamasaki T . Stimulation of fruit growth of kiwifruit, Actinidia chinensis Planch. by N-(2-chloro-4-pyridyl)-N\"-phenylurea, a diphenylurea-derivative cytokinin[J]. Scientia Horticulturae, 1988, 35(1):109-115.
[2] Zofia T. 2-Halo-4-nitropyridine [J]. Roczniki Chem.1962, 36:1313-1320.
[3] V. Krishnakumar, S. Dheivamalar, R. John Xavier. Analysis of vibrational spectra of 4-amino-2,6-dichloropyridine and 2-chloro-3,5-dinitropyridine based on density functional theory calculations[J]. Spectrochimica Acta Part A Molecular & Biomolecular Spectroscopy, 65(1):147-154.
[4] Wikaira, Jan L, Landee, Christopher P, Ludy, Sarah J. Tetrahalocuprate salts of substituted 4-aminopyridines: Synthesis, structure and magnetic properties[J]. Polyhedron, 52:770-780.
[5] Changshou W . Synthesis of 4-Amino-2-Chloropyridine[J]. SHAANXI CHEMICAL INDUSTRY, 1999.
[6] https://pubchem.ncbi.nlm.nih.gov/compound/84432
[7]https://www.chemspider.com/Chemical-Structure.21109299.html?rid=636951e1-ea86-4ce4-bcfa-ad31122cece5&page_num=0
- Related articles
- Related Qustion
- 4-Amino-2-chloropyridine: properties, applications and safety Nov 16, 2023
4-Amino-2-chloropyridine is a versatile compound used in pharmaceutical, agrochemical, and dye synthesis, but requires caution due to potential hazards.
Pyridine and its derivatives have been developed and utilized relatively early at home and abroad. It is widely used in medicine, pesticides, rubber, dyes and other fields.....
Dec 9,2019Drug IntermediateSildenafil, the first US FDA-approved, oral phosphodiesterase type-5 inhibitor, has revolutionized the treatment of erectile dysfunction sine its approval in 1998. Since sildenafil is a potent inhibitor of cyclic guanosine monophosphate.....
Dec 9,2019Hormones and the Endocrine System4-Amino-2-chloropyridine
14432-12-3You may like
- The benefits of Milk Thistle Seed Oil
Apr 16, 2024
- Abametapir:Introduction and Synthesis
Dec 25, 2023
- What is 1,8-Diazabicyclo[5.4.0]undec-7-ene?
Jul 15, 2020
4-Amino-2-chloropyridine manufacturers
- 4-Amino-2-chloropyridine
-
- 2025-12-15
- CAS:14432-12-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 4-Amino-2-chloropyridine
-

- $0.00 / 1KG
- 2025-12-15
- CAS:14432-12-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- 4-Amino-2-chloropyridine
-

- $0.00 / 25Kg/Drum
- 2025-12-15
- CAS:14432-12-3
- Min. Order: 100g/Bag
- Purity: 99%
- Supply Ability: 50mt