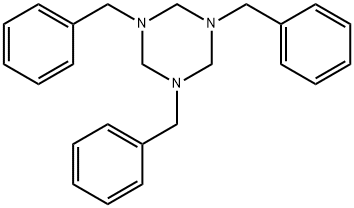
The synthesis of 1,3,5-Tribenzyl-1,3,5-triazinane
Dec 18,2019
1,3,5-Tribenzyl-1,3,5-triazinane is used as a reagent in the synthesis of phosphinic acid based N-Acetylated alpha-linked acidic dipeptidase (NAALADase) inhibitors, which may potentially be used for the treatment of both neurodegenerative disorders and peripheral neuropathies [1].
Let us see the synthesis routes of 1,3,5-tribenzyl-1,3,5-triazinane.
Takano Naoyuki and his coworkers described the process for 1,3,5-Tribenzyl-1,3,5-triazinane synthesis as shown in scheme 1 [2]. The step of reacting the halogen compound (benzyl chloride) with ammonia and formaldehyde to obtain the hexahydrotriazine compound 1,3,5-tribenzylhexahydro-1,3,5-triazine. The detail process was described as below. Into a glass autoclave, 25.57 parts by weight of benzyl chloride, 19.57 parts by weight of paraformaldehyde and 113.5 parts by weight of a 12 wt% ammonia/methanol solution were charged and reacted under stirring at an internal temperature of 40°C for 3 hours, 50°C for 2 hours and 70°C for 1 hour. The maximum value of the internal pressure (gauge pressure) during the reaction was 0.08MPa. The resulting reaction liquid and methanol rinse were transferred into a four-neck flask, subjected to reduced pressure to remove ammonium remaining in the reaction liquid, and further concentrated to remove methanol. To the residual liquid thus obtained was added 200 parts by weight of water, and methanol was distilled off together with water under reduced pressure. The residue was subjected to an extraction/separation treatment using 150 parts by weight of toluene to obtain 161.9 parts by weight of a toluene solution containing 1,3,5-Tribenzyl-1,3,5-triazinane.

Scheme 1 The synthesis of 1,3,5-tribenzyl-1,3,5-triazinane
Smiths and his team developed a route of 1,3,5-tribenzyl-1,3,5-triazinane synthesis with ~94% yield [3]. The synthesis route was shown in scheme 2. The procedure: To a round-bottomed flask (125 mL) equipped with a reflux condenser was added the appropriate amine (0.05 mmol), toluene (40 mL) and formaldehyde (37%, 4.1 mL). The solution was brought to reflux using an external oil bath and kept stirring for 30min. Then, the toluene was evaporated under reduced pressure, and the residue was dissolved in ethyl acetate and washed with a saturated aqueous solution of sodium chloride. After evaporation of the solvent under reduced pressure in a rotary evaporator, the residue was purified by silica gel column chromatography using hexane/ethyl acetate 9:1 as the eluent. After purification, the final product 1,3,5-tribenzyl-1,3,5-triazinane was obtained.

Scheme 2 The synthesis of 1,3,5-tribenzyl-1,3,5-triazinane with high yield
Reference
[1] https://www.trc-canada.com/product-detail/?T768950
[2] Takano Naoyuki etc., EP1961733 A1,
[3] Smith, Gary S.; Berlin, K. Darrell; Zisman, Stan A.; Holt, Elizabeth M.; Green, Vicki A.; Helm, Dick Van Der Phosphorus and Sulfur and the Related Elements, 1988, vol. 39, p. 91 - 11
- Related articles
- Related Qustion
Know from the recipe of Silm FX, 1,3,7,8-tetramethylxanthine is one of the components. The product claims it is useful for men and women who are striving to lose weight, but frequently fall short due to tiredness and unhealthy snack craving....
Dec 18,2019SupplementsThe N-methyl-d-aspartate (NMDA) receptor is arguably an important signaling mechanism in the human brain and NMDA receptors play a critical role in regulating the strength of synapses, that is, in regulating synaptic plasticity.....
Dec 18,2019Pharmaceutical intermediates1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE
2547-66-2You may like
1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE manufacturers
- 1,3,5-TRIBENZYLHEXAHYDRO-1,3,5-TRIAZINE
-

- $500.00 / 1KG
- 2019-07-06
- CAS:2547-66-2
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton