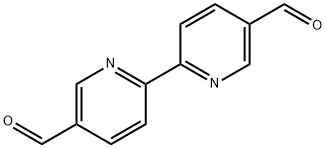
what is 2,2'-Bipyridine-5,5'-dicarboxaldehyde
Feb 19,2020
2,2'-Bipyridine-5,5'-dicarboxaldehyde is an important organic intermediate (building block) to synthetize substituted bipyridine products.
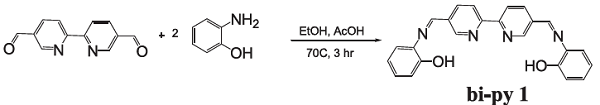
Suganya et al. reported [1] its application on the synthesis of bipy-1 and the binding property with Co(OAc/F)2, Ni(OAc/F)2, and Cu(OAc/F)2 manifested by colorimetric analysis and UV-vis titration. Bipy-1 showed selective recognition of dimethyl sulphoxide solution of tetrabutyl ammonium salt of F- ion accompanied with a UV-vis band at 529nm and interesting binding of aqueous Co, Ni, and Cu acetates/fluorides, as confirmed by distinct color changes from fluorescent green to pink or orange and a strong band around 480–510 nm in the UV-vis spectrum. In the presence of Co, Ni, and Cu countercations, any form of metal acetate/fluorides was found to be able to respond to similar color changes from fluorescent green to pink or orange, showing a band around 480–510 nm. This type of output clearly indicates that the in situ formation of Co, Ni, and Cu acetates/fluorides also coordinates with bipyridyl nitrogen atoms.
Lei et al. reported [2] its application on the synthesis of a diquat-containing macrocyclic anion acceptor. The macrocycle demonstrates promising abilities to recognize anions including HCO3-and HPO42- in water, as well as halides in an organic solvent. The driving forces include hydrogen bonding interactions, electrostatic interactions, and anion–p interactions, which take place in a cooperative manner.
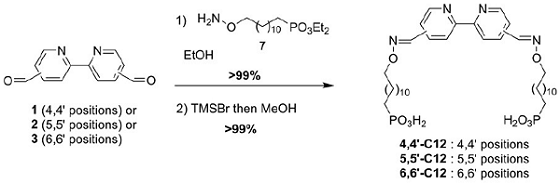
Forato et al. reported [3] its application on the synthesis of bipyridine hydrogenation catalysts. When one or two dodecylphosphonate tethers branched via a C=N-O connector were introduced at the 4,4’-, 5,5’-, or 6,6’-positions of the 2,2’-bipyridine backbone, the hydrogenation of 6-methyl-5-hepten-2-one under hydrogen pressure occurred preferentially at the C=C bond, consistent with the chemoselectivity found for the homogenous parent catalyst. The manner in which the phosphonate end groups are introduced into the 2,2’-bipyridine backbone (number of tethers and their location on the aromatic rings) was found to strongly influence the microstructure of the resulting supported catalysts (surface area, pore size distribution, etc.), which, in turn, affected the reaction rates. The accessibility and the arrangement of the phosphonate-functionalized catalytic complex at the surface of the inorganic support is therefore dependent on its structure.
References
1. Suganya S, Zo HJ, Park JS, Velmathi S. Colorimetric detection of in situ metal acetates and fluorides by a bipyridyl-linked Schiff base[J]. J. Mol. Recognit. 2014, 27: 689–695
2. Lei Y. et al. A diquat-containing macrocyclic anion acceptor in pure water[J]. Chem. Commun., 2019, 55:8297--8300
Forato F. et al. Phosphonate-Mediated Immobilization of Rhodium/Bipyridine Hydrogenation Catalysts[J]. Chem. Eur. J. 2018, 24:2457 – 2465
- Related articles
- Related Qustion
- 2,2'-Bipyridyl-5,5'-dialdehyde: properties, synthesis and safety Dec 14, 2023
2,2'-Bipyridyl-5,5'-dialdehyde is a versatile compound used in catalysis, and materials science due to its unique properties. Proper handling is required due to its irritant classification.
cis-4-Hydroxy-D-proline is a substrate that may be used to study the specificity and kinetics of D-alanine dehydrogenase.D-proline in which a hydrogen at the 4-position of the pyrrolidine ring is substituted by a hydroxy group.....
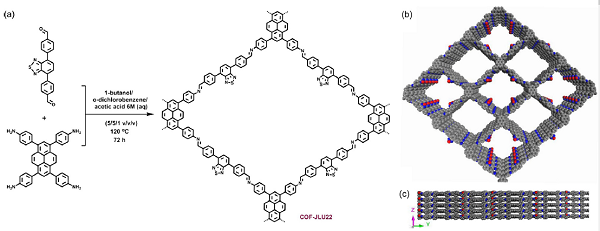
Feb 19,2020Amino Acids and DerivativesBenzenamine,4,4',4'',4'''-(1,3,6,8-pyrenetetrayl)tetrakis- is an important organic intermediate (building block) to synthetize covalent organic frameworks (COFs)....
Feb 19,2020Organic Synthesis Intermediate2,2'-Bipyridyl-5,5'-dialdehyde
135822-72-9You may like
2,2'-Bipyridyl-5,5'-dialdehyde manufacturers
- 2,2'-Bipyridine-5,5'-dicarboxaldehyde
-
- $48.00 / 1g
- 2025-12-12
- CAS:135822-72-9
- Min. Order: 1g
- Purity: 0.98
- Supply Ability: 25kg
- 2,2'-BIPYRIDYL-5,5'-DIALDEHYDE
-

- $1.00 / 1KG
- 2019-07-06
- CAS:135822-72-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100