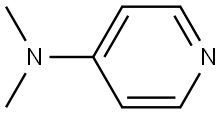
What is 4-Dimethylaminopyridine?
Jun 30,2020
General Description
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH3)2NC5H4N. This colourless solid is of interest because it is more basic than pyridine, owing to the resonance stabilisation from the NMe2 substituent.
Chemical Properties
DMAP (m.p. 112-113°C) and PPY (m.p. 57-58°C) are colorless, crystalline substances which are very soluble in methanol, ethyl acetate, chloroform, methylene chloride, 1,2-dichloroethane, acetone, and acetic acid and less soluble in cold hexane, cyclohexane, and water. DMAP can be recrystallized from ethyl acetate and PPY from pentane or hexane. The basicities of DMAP and PPY in water as well as the dipole moment of DMAP in benzene and dioxane have been determined by several groups. Of especial interest are the thermodynamic investigations concerned with the protonation of DMAP in water and calculations whereby the influence of substituents on the basicity has been determined.
Reactions
The functional groups and class of compounds that are involved in the reactions with DMAP include alcohols, amines, arenes, azides, carbenes, enols, epoxides, hydrazines, hydroxylamines, phenols, thiols, lipids, sugars, aminoacids, peptides, alkaloids, steroids, terpenes, and others. Reactions that have been published in the literature using DMAP fall into, but are not limited to, the following types of reactions: Acylation; Acetylation; Alkylation; Benzoylation; Bischler-Naperalski cyclization, Carbonylation; Carbo- diimidation; Cyclization; Dehydration; Esterificaton; Indole Synthesis; Nucleophilic Substitution; Rearrangement; Silylation; Sulfonamidation; Sulfonation; Tritylation; Formylation; Carbamoylation; Phosphorylation; Lactonization; Pivaloylation; Dakin-West Reaction; Baylis-Hillman Reaction.
DMAP reacts readily with electrophilic reagents. It is possible to quaternize DMAP in high yield with either methyl iodide or ethyl bromide, decomposes quantitatively in the presence of aqueous alkali to N-methy1-4-pyridone.
Addition of DMAP to S, S'-diethyl-S, S'-dimethyl-S, S-1, 2vinylenedisulfonium salts results in the smooth formation of the salt with concomitant generation of ethyl methyl sulfide.
Reaction of DMAP with acetylenedicarboxylic acid leads spontaneously to the bis-adduct in high yields.
On reaction with perbenzoic acid the strongly polar N-oxide is formed. Nitration of DMAP with HNO3/H2SO4 gives the 3-nitro derivatives in 81 % yield and, under forcing condition; the 3,5- dinitro compounds are obtained. Reaction with O-(p-toluenesulfony1) hydroxylamine affords the N-amino compound in 67% yield which is isolated as the perchlorate. By treatment with D2O it is possible to selectively exchange the a-protons in DMAP, with DClO4 to exchange the P-protons to furnish and with D2O/NaOD to replace all aromatic protons by deuterium.
Application as acylation catalysts
4-Dialkylaminopyridines were soon found to have general applicability for catalysis of a wide variety of reactions. 4-dimethylaminopyridine ’s (DMAP) wide applicability has been frequently reviewed since the first review appeared in 1978. The accelerating pace of reported applications for DMAP and the availability of DMAP in commercial quantities, at modest prices, have continued to stimulate great interest in its use as a catalyst in the fields of organic, polymer, analytical and biochemistry. Today there are thousands of examples of the use of DMAP in far ranging fields of chemistry in both patents and the research literature. Many full-scale production processes utilizing DMAP have been and are being operated. Several pharmaceutical and agricultural products that rely on DMAP’s superior catalytic properties in their synthetic sequences have been produced for years. Since 1976 more than 11,000 US patents have been granted which mention DMAP or dimethylaminopyridine.
Acylation of alcohol
The high catalytic activity of DMAP and PPY can be used for acylating sterically hindered secondary or tertiary alcohols with carboxylic anhydrides or acyl halides when the pyridine method fails. In most cases, it is necessary to use only 0.05-0.2 mol of catalyst per mol of substance and the acid that is formed can be bound with an equivalent amount of trimethylamine[21, 22] or pyridine[20]. Such solvents as hexane, toluene, benzene, methylene chloride, chloroform, ethyl acetate, tetrahydrofuran, triethylamine, pyridine, or acetic anhydride are suitable for use with these catalysts.
Among the tertiary alcohols which can be easily acylated with DMAP and PPY, mention should be made of l-methyl cyclohexanol, 1- ethynylcyclohexanol, 1,l-diphenylethanol, linalool, l, l-dimethoxy-2 -methyl-3-buten-2-ol, 5,5-dimethoxy-2-methyI-3-pentyn-2-ol, and cis- 4- (1-hydroxyisopropyl)-2-methylcyclohexanone.
Acylation of phenols
In the acylation of phenols, DMAP and PPY effect a similar increase in reaction rate as is found in the case of alcohols. Hence, the method is of interest for the acylation of sterically hindered phenols. For example, mesitol can be smoothly acetylated with acetic anhydride/DMAP to 2,5-ditert-butylphenol and analogous compounds can be transformed into acyl derivatives of the type in high yields. 11,12-Dihydroglaziovine smoothly affords the acyl derivative.
Acylation of amines
DMAP and PPY have been seldom used for the acylation of amines. The kinetic investigations of Lituinenko and Kirichenko have shown that an enormous increase in reaction rate is observed when acylations are carried out in aprotic solvents. These authors have determined the following relative rate constants (in parentheses) for the amine- catalyzed acylation of m-chloroaniline with benzoyl chloride in benzene: N, N-dimethylaniline (0.1); triethylamine (0.072); 2,6- dimethylpyridine (0.03); pyridine (1.80); 4-methylpyridine (10.0); and DMAP (10600).
Acylation of enolates
Acylations involving CH-acid compounds which can be performed with pyridine or triethylamine as catalyst are found to proceed at a much higher rate when DMAP or PPY is used. The Dakin-West reaction of N- acyl amino acids, in which a 2-oxazolin-5-one is acetylated at C-4 with a carboxylic anhydride in pyridine with formation of a new C-C bond, has been extensively investigated. The combination products, consisting of the ambident oxazolin-5-one anions and N-acylpyridinium cations initially formed under kinetic control, are transformed via the ion pair into the thermodynamically most stable product.
Decarboxylative ring opening by the subsequently formed carboxylic acid yields the a-acyl amino ketone.
Reactions of isocyanates
Pyridine-catalyzed reactions of isocyanates with carboxylic acids to form amides are found to be strongly accelerated on replacement of pyridine by DMAP. Phenylacetic acid is found to react with phenyl isocyanate in 1,2-dichloroethane at 24°C to give the amide in 66 % yield in less than 5 min; whereas on using the same amount of pyridine only 53% could be isolated after 2h. With triethylamine, only very little is formed besides diphenylurea.
Miscellaneous Applications
DMAP has been used in the hardening of epoxy resins with dicyanodiamine, in the transformation of nitriles into thionamides, and in the transfer of silyl groups to tertiary hydroxyl groups.
Transfer of Functional Groups
Dimethylarninopyridinium salts are interesting reagents for the transfer of acyl and also cyano and phosphono groups in aqueous medium.
- Related articles
- Related Qustion
- Review and related research on 4-Dimethylaminopyridine Mar 4, 2024
4-Dimethylaminopyridine is a highly versatile nucleophilic catalyst for acylation reactions and esterifications.
- 4-Dimethylaminopyridine: An Effective Acyl Transfer Agent Apr 14, 2023
4-Dimethylaminopyridine is a derivative of pyridine with the chemical formula (CH3)2NC5H4N.
N,N-Diisopropylethylamine is also known as Hunig’s base and abbreviated as DIPEA or DIEA, N,N-Diisopropylethylamine is a sterically hindered amine and an organic compound.....
Jun 29,2020AmidesIsopropanol is also known as isopropyl alcohol. It is the simplest secondary alcohol and is one of the isomers of n-propanol. It is a kind of flammable liquid which is colorless with strong smell.....
Jul 1,2020Organic Chemistry4-Dimethylaminopyridine
1122-58-3You may like
4-Dimethylaminopyridine manufacturers
- 4-Dimethylaminopyridine
-
- 2025-12-20
- CAS:1122-58-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 4-Dimethylaminopyridine(DMAP)
-

- $0.00 / 1kg
- 2025-12-19
- CAS:1122-58-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20MT
- 4-Dimethylaminopyridine
-

- $5.00 / 25kg
- 2025-12-18
- CAS:1122-58-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100mt