
1,1,1,3,3,3-hexafluoro-2-(4-iodophenyl)propan-2-ol synthesis
- Product Name:1,1,1,3,3,3-hexafluoro-2-(4-iodophenyl)propan-2-ol
- CAS Number:1214788-34-7
- Molecular formula:C9H5F6IO
- Molecular Weight:370.03
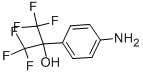
722-92-9
131 suppliers
$8.00/1g

1214788-34-7
1 suppliers
inquiry
Yield:1214788-34-7 88%
Reaction Conditions:
Stage #1: 4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenylaminewith NaNO2 in lithium hydroxide monohydrate;N,N-dimethyl-formamide at 0; for 0.25 h;
Stage #2: with hydrogenchloride in lithium hydroxide monohydrate;N,N-dimethyl-formamide at 0; for 1.25 h;
Stage #3: with potassium iodide in lithium hydroxide monohydrate;N,N-dimethyl-formamide at 0 - 20;
Steps:
1.A Step A:
To a solution of 1 (15 g, 1.0 eq) in DMF (120 mL) was added a solution of NaN02 (4.4 g, 1.1 eq) in water (30 mL). The mixture was cooled to 0 °C for 15 minutes and 6N HCl (29 mL, 3.0 eq) was added dropwise to the reaction mixture for over 15 minutes at 0 °C. The resulting mixture was stirred at 0 °C for lh. KI (10.1 g, 1.05 eq) was added with portions (over 15 minutes). The reaction mixture was stirred at 0 °C for lh, and then stirred at room temperature overnight. The reaction mixture was diluted with water (-500 mL) and extracted with EtOAc/Hexane (2: 1, 3x150 mL). The combined organic phase was washed with NaHS03, water, and brine. The crude mixture was purified on a silica gel column to afford 2 (18.85 g, 88%) as a pale yellow oil.
References:
WO2018/81558,2018,A1 Location in patent:Paragraph 00148
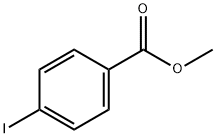
619-44-3
318 suppliers
$6.00/10g
81290-20-2
416 suppliers
$13.00/1g

1214788-34-7
1 suppliers
inquiry
23516-84-9
57 suppliers
$71.00/100mg