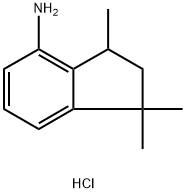
1,1,3-Trimethyl-indan-4-ylamine hydrochloride synthesis
- Product Name:1,1,3-Trimethyl-indan-4-ylamine hydrochloride
- CAS Number:1616291-21-4
- Molecular formula:C12H18ClN
- Molecular Weight:211.73
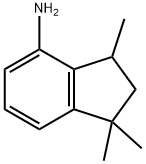
94568-76-0
23 suppliers
inquiry

1616291-21-4
17 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in water;toluene at 70; for 1 h;Inert atmosphere;
Steps:
2
References:
EP2940001,2015,A1 Location in patent:Paragraph 0053; 0054; 0055; 0056; 0057-0060; 0063-0064