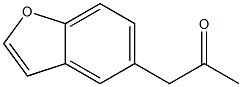
1-(1-benzofuran-5-yl)propan-2-one synthesis
- Product Name:1-(1-benzofuran-5-yl)propan-2-one
- CAS Number:286836-32-6
- Molecular formula:C11H10O2
- Molecular Weight:174.1959
23145-07-5
273 suppliers
$15.00/1g
108-22-5
267 suppliers
$20.00/25mL

286836-32-6
9 suppliers
inquiry
Yield:286836-32-6 96%
Reaction Conditions:
with tributyltin methoxide;tris-(o-tolyl)phosphine;palladium (II) chloride in toluene at 100; for 16 h;Inert atmosphere;
Steps:
2.1 Step 1:
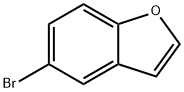
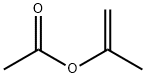
To a stirred solution of 5-bromobenzofuran (5-1) (20 g, 101.52 mmol, 1 eq.) in dry toluene (400 mL) was added tri(o-tolyl)phosphine (1.84 g, 6.09 mmol, 0.06 eq.), tributyl tin methoxide (48.89 mL, 152.28 mmol, 1.5 eq.) and Isopropenyl acetate (16.99 mL, 156.34 mmol, 1.54 eq.) then the resulting reaction mixture was degassed under nitrogen for 15 minutes. Then palladium (II) chloride (1.26 g, 7.10 mmol, 0.07 eq.) was added to the reaction mixture and the resulting reaction mixture was heated to 100°C for 16 h. Upon completion, monitored by TLC (10% EA in Hexane), the reaction mixture was cooled to RT, evaporate under vacuum. Then the residue was dissolved in ethyl acetate and filtered through celite bed, washed with water, and saturated potassium fluoride solution, followed by brine solution. Combined organic layer was dried over anhydrous sodium sulphate, solvent was removed under vacuum and purified by silica gel column chromatography using ethyl acetate/hexane (10:90 v/v) as eluent to afford 1- (benzofuran-5-yl)propan-2-one (5-3) as light yellow gum (17 g, 96%).1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 2.0 Hz, 1H), 7.53 (d, J = 8.48 Hz, 1H), 7.46 (s, 1H), 7.13 (dd, J = 1.52 Hz, 8.44 Hz, 1H), 6.92 (bs, 1H), 3.83 (s, 2H), 2.12 (s, 3H). LCMS: (ES) C11H10O2 requires 174, found 175 [M + H]+.
References:
WO2021/252538,2021,A2 Location in patent:Page/Page column 274; 286; 287; 306