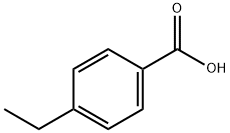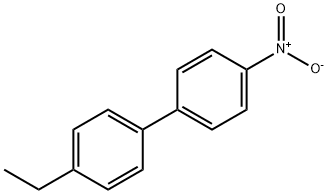
1,1'-Biphenyl, 4-ethyl-4'-nitro- synthesis
- Product Name:1,1'-Biphenyl, 4-ethyl-4'-nitro-
- CAS Number:279242-09-0
- Molecular formula:C14H13NO2
- Molecular Weight:227.26
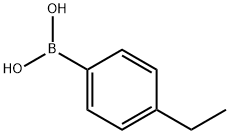
636-98-6
36 suppliers
$17.00/5g
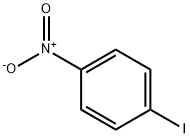
63139-21-9
289 suppliers
$5.00/250mg

279242-09-0
3 suppliers
inquiry
Yield:279242-09-0 99%
Reaction Conditions:
with trimethyl-(2-hydroxyethyl)ammonium chloride;potassium carbonate in glycerol at 80; for 1 h;Sealed tube;Suzuki Coupling;
Steps:
2.6. Catalytic Suzuki coupling reaction
General procedure: Typically, a 10 mL screw-capped vial was charged with arylboronicacid (1.1 mmol), aryl halide (1 mmol), K2CO3 (2 mmol),GO/Fe3O4G2/Co (1 mg, 0.4 mol %), and DES (ChCl:glycerol,3 mL). Afterward, the vial was put at 80 C to form the correspondingcoupling products (monitored by TLC). After completing thereaction, the catalyst was collected with a magnet and the reactionmixture was quenched with water and extracted with ethyl acetate(3 5 mL). The organic layer was dried with MgSO4 and evaporatedunder vacuum to yield the crude product, which was thenpurified by column chromatography using silica gel. Finally, theproducts were subjected to structural identification by 1H and13C NMR spectroscopies.
References:
Masteri-Farahani, Majid;Niakan, Mahsa [Journal of Molecular Liquids,2022,vol. 355,art. no. 118932]

63139-21-9
289 suppliers
$5.00/250mg
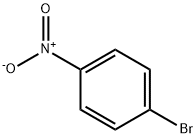
586-78-7
18 suppliers
$14.00/5g

279242-09-0
3 suppliers
inquiry

63139-21-9
289 suppliers
$5.00/250mg
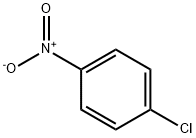
100-00-5
53 suppliers
$14.00/25g

279242-09-0
3 suppliers
inquiry

13629-88-4
0 suppliers
inquiry

279242-09-0
3 suppliers
inquiry