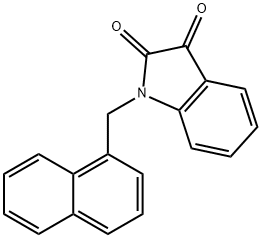
1-(1-naphthylmethyl)-1H-indole-2,3-dione synthesis
- Product Name:1-(1-naphthylmethyl)-1H-indole-2,3-dione
- CAS Number:79183-21-4
- Molecular formula:C19H13NO2
- Molecular Weight:287.31
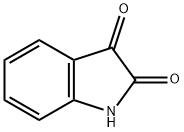
91-56-5
432 suppliers
$5.00/25g
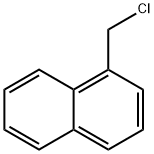
86-52-2
334 suppliers
$9.00/10g

79183-21-4
12 suppliers
$158.00/500mg
Yield:79183-21-4 61%
Reaction Conditions:
Stage #1: indole-2,3-dionewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.166667 h;
Stage #2: 1-Chloromethylnaphthalene in N,N-dimethyl-formamide at 0 - 20; for 12.5 h;
Steps:
Intermediate 59: 1-(Naphthalen-1-ylmethyl)indoline-2,3-dione:
Indoline-2,3-dione (37 g, 0.25 mol) was dissolved in DMF (740 ml) and cooled to 0°C. Sodium hydride was added to the above mixture in portions at 0°C and stirred at same temperature for 10 mins. 1-(Chloromethyl)naphthalene was added to above reaction mixture at 0°C, stirred at 0°C for 30 mins and at rt for 12 h. After 12 h, reaction mixture was quenched with satd. NH4CI (1 It) to obtain a solid. Solid was filtered and washed with water (500 ml). Solid was dissolved in MeOH and DCM (1:9) mixture (2 It) and dried on anhydrous Na2SO4. This solution was distilled to obtain a crude. Crude was purified by column chromatography on 60-120 mesh silica gel using DCM as eluent. Combined pure fractions from column were distilled to obtain titled compound (43.8 g) as a brown solid. Yield: 61%. 1H-NMR (δ ppm, DMSO-d6, 400 MHz): 8.20 (d, J 8.4, 1H), 7.99 (d, J 8.4, 1H), 7.88 (d, J 8.4, 1H), 7.68-7.50 (m, 5H), 7.42 (t, J 8, 1H), 7.12 (t, J 7.6, 1H), 6.90 (d, J 8, 1H), 5.39 (s, 2H).
References:
WO2021/220120,2021,A1 Location in patent:Paragraph 206

91-56-5
432 suppliers
$5.00/25g
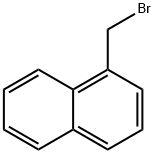
3163-27-7
187 suppliers
$7.00/1g

79183-21-4
12 suppliers
$158.00/500mg