
1,10-DIAMINODECANE synthesis
- Product Name:1,10-DIAMINODECANE
- CAS Number:646-25-3
- Molecular formula:C10H24N2
- Molecular Weight:172.31
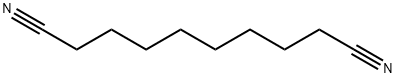
1871-96-1

646-25-3
The general procedure for synthesizing decanediamine from decanedinitrile is as follows: Example 2: Hydrogenation of decanedinitrile 50 g of 1,12-decanediamine and 50 g of water were added to a 750 ml autoclave equipped with a self-priming turbine stirrer. Subsequently 10g of Raney nickel catalyst doped with 2 wt% Cr and alkalized with sodium hydroxide was added. The reactor was displaced first with nitrogen and then twice with hydrogen, the hydrogen pressure being maintained at 10 bar. The reaction mixture was heated to 90°C and the reaction was carried out at a hydrogen pressure of 20 bar. Decanedinitrile prepared in Example 1 was slowly added via a pump and a total of 400 g was injected over a period of 4 h. The heat generated during the reaction was maintained at a constant temperature by a cooling system. After the addition of sebacic nitrile was completed, the reaction was continued for 15 minutes at 20 bar hydrogen and 90°C. Upon completion of the reaction, the mixture was cooled to room temperature and the gas in the reactor was replaced with nitrogen. The catalyst was removed by filtration and part of the catalyst was recycled. The catalyst deactivation criterion was 1 g of nickel corresponding to 1 kg of hydrogenated decanedinitrile. Finally, the decanediamine is purified by distillation under reduced pressure to collect the fraction at 140 °C and 10 mmHg, which solidifies at 62 °C. The yield of this process was 95%. Note: In order to ensure that the reaction yield is maximized, the dosing rate of sebacodinitrile needs to be controlled to avoid its accumulation in the reaction system.

1871-96-1
134 suppliers
$20.37/5g

646-25-3
280 suppliers
$6.00/25g
Yield:646-25-3 95%
Reaction Conditions:
with hydrogen;sodium hydroxide in lithium hydroxide monohydrate at 90; under 15001.5 Torr; for 4.25 h;
Steps:
2 Example 2 Sebaconitrile Hydrogenation
Example 2 Sebaconitrile Hydrogenation [0060] 50 g of 1,12-decanediamine and 50 g of water are introduced into a 750-ml autoclave equipped with a self-aspirating turbine. 10 g of Raney nickel doped with 2% by weight of Cr and basified with sodium hydroxide are introduced. The reactor is flushed with nitrogen and then twice with hydrogen, with 10 bar of pressure. Heating is carried out under a hydrogen pressure of 20 bar at 90° C. The sebaconitrile of example 1 is then introduced with a pump; 400 g of dinitrile are injected of the course of 4 hours. The reaction is exothermic. The temperature is kept constant by cooling. After the end of the injection, the mixture is left at 90° C. for 15 min under 20 bar of hydrogen. The mixture is brought back to ambient temperature and the reactor is flushed with nitrogen. The catalyst is filtered off and partially recycled. A catalyst deactivation of 1 g of nickel for 1 kg of hydrogenated dinitrile is accepted. The diamine is then purified by distillation under reduced pressure. The diamine is recovered at 140° C. under 10 mmHg, the latter solidifies at 62° C. The yield is 95%. In order to have a maximum reaction yield, it is necessary to supply the nitrile at a sufficient flow rate without accumulating the nitrile in the reaction medium.
References:
US2013/204001,2013,A1 Location in patent:Paragraph 0060
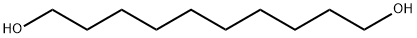
112-47-0
338 suppliers
$6.00/25g

646-25-3
280 suppliers
$6.00/25g
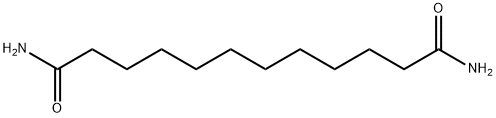
6224-99-3
10 suppliers
inquiry

646-25-3
280 suppliers
$6.00/25g
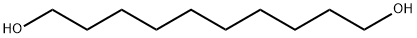
112-47-0
338 suppliers
$6.00/25g

23160-46-5
79 suppliers
$75.00/250mg

646-25-3
280 suppliers
$6.00/25g
17607-18-0
4 suppliers
inquiry

646-25-3
280 suppliers
$6.00/25g