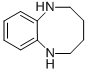
1,2,3,4,5,6-HEXAHYDRO-BENZO[B][1,4]DIAZOCINE synthesis
- Product Name:1,2,3,4,5,6-HEXAHYDRO-BENZO[B][1,4]DIAZOCINE
- CAS Number:39161-58-5
- Molecular formula:C10H14N2
- Molecular Weight:162.23
![2,3-dihydro-1H-pyrrolo[1,2-a]benzimidazole](/CAS/GIF/7724-48-3.gif)
7724-48-3
17 suppliers
inquiry
21627-58-7
45 suppliers
$58.00/250mg
![1,2,3,4,5,6-HEXAHYDRO-BENZO[B][1,4]DIAZOCINE](/CAS/GIF/39161-58-5.gif)
39161-58-5
8 suppliers
$824.66/1G
Yield:39161-58-5 35% ,21627-58-7 43%
Reaction Conditions:
Stage #1: 2,3-dihydro-1H-pyrrolo[1,2-a]benzimidazolewith diisobutylaluminium hydride in toluene at 0; for 23 h;Inert atmosphere;Reflux;
Stage #2: with water;sodium fluoride in toluene;benzene at 0 - 20; for 0.333333 h;Inert atmosphere;regioselective reaction;
Steps:
Generalprocedure for the preparation of 3 and 4
General procedure: To a solution of 2a (0.5 g, 3.17mmol) in dry toluene (10 mL) cooled to 0°C was added a solution of DIBAH (2.70 g, 19 mmol) in dry toluene (6 mL) dropwise with stirring under argon.The reaction mixture was stirred for7 hours and then boiled under reflux for 16 hours. Benzene (40mL) was added to the reaction mixture. It was cooled to 0°C and NaF (3.19 g)was added portionwise with stirring and then water (1.03 mL) was added dropwise. The resulting suspension was stirred for 20 min at room temperature. The amorphous precipitate was filtered, washed with CH2Cl2 (2*30 mL). Evaporation of the solvent afforded oil that waspurified by column chromatography to give 3a as a red oil (0.22 g, 43%)and 4a as beige crystals (0.18 g, 35%).
References:
Shvidenko, Tatyana;Nazarenko, Kostiantyn;Shvidenko, Konstantin;Kostyuk, Aleksandr [Tetrahedron Letters,2014,vol. 55,# 1,p. 279 - 281] Location in patent:supporting information
![1,6-Benzodiazocine, 1,2,3,4,5,6-hexahydro-1,6-bis[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/39161-59-6.gif)
39161-59-6
0 suppliers
inquiry
![1,2,3,4,5,6-HEXAHYDRO-BENZO[B][1,4]DIAZOCINE](/CAS/GIF/39161-58-5.gif)
39161-58-5
8 suppliers
$824.66/1G