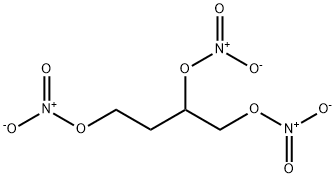
1,2,4-BUTANETRIOL TRINITRATE synthesis
- Product Name:1,2,4-BUTANETRIOL TRINITRATE
- CAS Number:6659-60-5
- Molecular formula:C4H7N3O9
- Molecular Weight:241.11
3068-00-6
284 suppliers
$10.00/5g

6659-60-5
8 suppliers
inquiry
Yield:6659-60-5 80%
Reaction Conditions:
with ammonium nitrate;sulfuric acid at 15; for 3 h;Cooling with ice;
Steps:
5
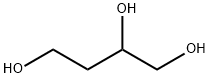
Example 5; Nitration of 1,2,4-Butanetriol (BT) to form 1,2,4-Butanetriol Trinitrate (BTTN); A round-bottom flask was charged with a magnetic stir bar and 25 mL of sulfuric acid, and then chilled in an ice bath. Ammonium nitrate (10.76 g (134.4 mmol)) was added at a rate such that the temperature of the nitrating solution did not exceed 15° C. BT (2 mL (22.4 mmol), 95% purity, purchased from Sigma-Aldrich Co.) was added at a rate such that the temperature of the reaction mixture did not exceed 15° C. Once all the BT was added and the temperature of the reaction mixture began to decrease, the ice bath was removed and the reaction was stirred for 3 hours at ambient conditions. The reaction mixture was added in one portion to 100 g crushed ice and stirred gently until all the ice melted. The resulting emulsion was transferred to a separatory funnel and extracted four times with 25 mL methylene chloride. The combined organic extracts were dried with MgSO4 and then concentrated to produce BTTN as a clear pale yellow liquid (4.3 g of BTTN, an 80% yield). The reaction is shown below: The BTTN was characterized by 1H and 13C NMR spectroscopy and HPLC. As shown in FIG. 5, the BTTN was about 97% pure. The 1H and 13C NMR spectra (not shown) confirmed the synthesis of BTTN.
References:
US2012/130115,2012,A1 Location in patent:Page/Page column 5