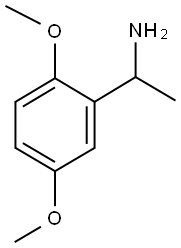
1-(2,5-DIMETHOXY-PHENYL)-ETHYLAMINE synthesis
- Product Name:1-(2,5-DIMETHOXY-PHENYL)-ETHYLAMINE
- CAS Number:35253-26-0
- Molecular formula:C10H15NO2
- Molecular Weight:181.23
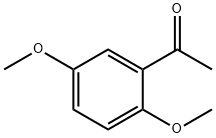
1201-38-3
333 suppliers
$6.00/5g

35253-26-0
34 suppliers
$60.00/1g
Yield:35253-26-0 75%
Reaction Conditions:
with ammonium hydroxide;ammonium acetate;sodium cyanoborohydride;zinc in ethanol;water at 80; for 36 h;
Steps:
4.2.1. General procedure for the synthesis of amines via reductive amination of acetophenones
General procedure: To a saturated solution of NH4OAc in EtOH (40 mL) were added activated Zn (5 equiv), acetophenone (1 equiv), NaBCNH3(3 equiv) and 30% aq NH3 (10 mL) respectively. The mixture wasstirred at 80 C for 36 h. The reaction mixture was cooled to rt and concentrated under reduced pressure. The residue was dissolved in CH2Cl2 and made basic using 1 M NaOH (50 mL). Theorganic phase was separated and aqua phase was extracted with (2x25 mL) CH2Cl2. The organic phases were combined and acidified with HCl (pH: 2.0). The organic layer was separated andH2O layer was extracted with CH2Cl2 (2 25 mL). The H2O layer was made basic with NaOH (pH: 10.0), The organic layer was extracted with CH2Cl2 (3 25 mL). Combined organic layers weredried over Na2SO4 and evaporation of the solvent afforded thedesired amines.
References:
Akincio?lu, Akin;Akincio?lu, Hülya;Gül?in, Ilhami;Durdagi, Serdar;Supuran, Claudiu T.;G?ksu, Süleyman [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 13,art. no. 12231,p. 3592 - 3602]