
1,2,5-thiadiazol-3-ylmethanol synthesis
- Product Name:1,2,5-thiadiazol-3-ylmethanol
- CAS Number:90507-33-8
- Molecular formula:C3H4N2OS
- Molecular Weight:116.14

89322-83-8
1 suppliers
inquiry

90507-33-8
4 suppliers
inquiry
Yield:90507-33-8 69%
Reaction Conditions:
Stage #1: [1,2,5]thiadiazole-3-carboxylic acid ethyl esterwith lithium borohydride in tetrahydrofuran at 20; for 18 h;
Stage #2: with water;ammonium chloride in tetrahydrofuran;
Steps:
33
Mix [1,2,5]thiadiazole-3-carboxylic acid (6.00 g, 46.1 mmol) and oxalyl chloride (11.7 g, 8.04 mL, 92.2 mmol) in CH2Cl2 (150 mL). To this heterogeneous slurry, add 10 drops of DMF and stir at room temperature. The reaction mixture will bubble and gradually become translucent within one hour. After one hour, concentrate the reaction mixture in vacuo to give the acid chloride as a brown oil.Dissolve the acid chloride in EtOH (50 mL) and stir at room temperature for one hour, then concentrate in vacuo to give the ethyl ester as a brown oil.Dissolve the ethyl ester in THF (100 mL) and add LiBH4 (2.0 M solution in THF, 46.1 mL, 92.2 mmol). Stir the reaction mixture at room temperature for 18 h. Pour the reaction mixture into aqueous NH4Cl (400 mL) and extract into EtOAc (3×150 mL). Dry the organics (MgSO4) and concentrate in vacuo to give 4.41 g crude product as a yellow oil. Purify on silica gel (40 g) using 30% EtOAc/hexanes to give 3.71 g (69%) of the title compound as a yellow oil. 1H-NMR (CDCl3) δ 8.59 (s, 1H), 5.00 (s, 2H).
References:
US2010/69404,2010,A1 Location in patent:Page/Page column 15
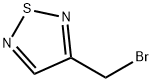
53012-70-7
5 suppliers
inquiry

90507-33-8
4 suppliers
inquiry
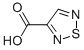
13368-86-0
59 suppliers
$150.00/1g

90507-33-8
4 suppliers
inquiry