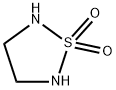
[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE synthesis
- Product Name:[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE
- CAS Number:5823-51-8
- Molecular formula:C2H6N2O2S
- Molecular Weight:122.15
![2-BENZYL-[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/144432-72-4.gif)
144432-72-4
![[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/5823-51-8.gif)
5823-51-8
General procedure for the synthesis of 1,2,5-thiadiazolidine 1,1-dioxide from 2-benzyl-1Λ~6~,2,5-thiadiazolidine-1,1-dione: A mixture of 2-benzyl-1,2,5-thiadiazolidine-1,1-dioxide (1.000 g, 4.7 mmol) with 20% palladium hydroxide (0.200 g) in methanol (20 mL) was placed under hydrogen atmosphere (1 atm) and stirred for 16 hours. Upon completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was concentrated to afford the white solid product 1,2,5-thiadiazolidine-1,1-dioxide (0.570 g, 99% yield). The product was characterized by 1H NMR (300 MHz, d6-DMSO): δ 6.68 (s, 2H), 3.30-3.25 (m, 4H).
![2-BENZYL-[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/144432-72-4.gif)
144432-72-4
18 suppliers
$560.32/1g
![[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/5823-51-8.gif)
5823-51-8
75 suppliers
$175.00/100mg
Yield: 99%
Reaction Conditions:
with water;palladium(II) hydroxide in methanol under 760.051 Torr; for 16 h;
Steps:
100 Preparation 100:1,2,5-Thiadiazolidine-1,1-dioxide
Preparation 100:1,2,5-Thiadiazolidine-1,1-dioxide [0437] [0438] A mixture of 2-benzyl-1,2,5-thiadiazolidine-1,1-dioxide (1.000 g, 4.7 mmol) and 20% palladium hydroxide (0.200 g) in methanol (20 mL) was stirred under a hydrogen atmosphere (1 atm) for 16 hrs. The mixture was filtered through Celite and the filtrate concentrated to afford a white solid (0.570 g, 99%). 1H NMR (300 MHz, d6-DMSO) δ 6.68 (s, 2H), 3.30-3.25 (m, 4H).
References:
Bersot, Ross;Humphries, Paul US2013/303524, 2013, A1 Location in patent:Paragraph 0437-0438
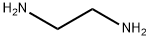
107-15-3
0 suppliers
$11.19/25ML
![[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/5823-51-8.gif)
5823-51-8
75 suppliers
$175.00/100mg
7803-58-9
352 suppliers
$6.00/10g
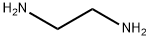
107-15-3
0 suppliers
$11.19/25ML
![[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/5823-51-8.gif)
5823-51-8
75 suppliers
$175.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![[1,2,5]THIADIAZOLIDINE 1,1-DIOXIDE](/CAS/GIF/5823-51-8.gif)
5823-51-8
75 suppliers
$175.00/100mg