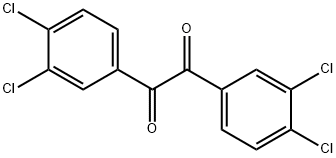
1,2-Bis(3,4-dichlorophenyl)ethane-1,2-dione synthesis
- Product Name:1,2-Bis(3,4-dichlorophenyl)ethane-1,2-dione
- CAS Number:74417-18-8
- Molecular formula:C14H6Cl4O2
- Molecular Weight:348.01
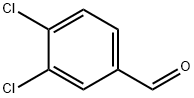
6287-38-3
264 suppliers
$8.00/10g

74417-18-8
5 suppliers
inquiry
Yield:74417-18-8 54.4 g (0.156 mol, 31%)
Reaction Conditions:
in methanol;water;nitric acid;toluene;
Steps:
7 EXAMPLE 7
EXAMPLE 7 Preparation of 3,3',4,4'-tetrachlorobenzil 241.3 g (1.4 mol) of 3,4-dichlorobenzaldehyde were placed in 125 ml of methanol, and 5 g of potassium cyanide in 7 g of water were added dropwise over 30 minutes under argon at 70° C. (immediate red coloration). The mixture was refluxed at this temperature for 4 h. The mixture was cooled, after which a clear red solution was obtained. 100 g of solvent were removed on a rotary evaporator to leave 293.8 g of a tough vitreous residue. The residue was admixed with 600 ml of toluene and heated at 60° C. with stirring. The suspension was then filtered at 45° C. and the filter cake was washed 2 times with 100 ml of toluene. Insoluble material was filtered off after 24 h and the filtrate was extracted by shaking three times with 200 ml of 20% strength sodium hydrogen sulfite solution. The solution was concentrated in vacuo to leave 194.1 g of residue. 140 g of the residue were placed in a vessel at 98° C. and 140 ml of 70% strength nitric acid were added dropwise to it over 2 h. After dilution at boiling temperature with 140 g of water, the mixture was allowed to cool slowly. After having cooled to 0° C., the mixture was filtered and washed with 200 g of water. This yielded 144.2 g of crude product which was recrystallized from 400 ml of ethanol. This yielded 54.4 g (0.156 mol, 31%) of 3,3',4,4'-tetrachlorobenzil and a further 24.4 g (0.07 mol, 14%) of this yellow compound in the form of crystal needles by concentration of the mother liquor. It was possible to improve the melting point of the product fractions by multiple recrystallization, from ethanol, from 165°-167.5° C. to 193°-198° C.
References:
US5463135,1995,A

113541-06-3
0 suppliers
inquiry

74417-18-8
5 suppliers
inquiry