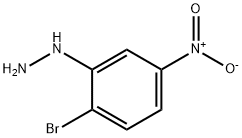
1-(2-bromo-5-nitrophenyl)hydrazine synthesis
- Product Name:1-(2-bromo-5-nitrophenyl)hydrazine
- CAS Number:100367-78-0
- Molecular formula:C6H6BrN3O2
- Molecular Weight:232.03
10403-47-1
201 suppliers
$22.00/5g

100367-78-0
10 suppliers
inquiry
Yield:100367-78-0 51%
Reaction Conditions:
Stage #1: 2-bromo 5-nitroanilinewith hydrogenchloride;sodium nitrite in water at 0; for 3 h;
Stage #2: with hydrogenchloride;tin(ll) chloride in water at 20; for 2 h;
Steps:
23.1 [0467] Step 1: (2-Bromo-5-nitrophenyl)hydrazine
[0468] To a suspention of 2-bromo-5-nitroaniline (1 g, 4.6 mmol) in cone. HCl (10 mL) at 0 DCwas slowly added a solution of NaN02 (382 mg, 5.5 mmol) in water (1.5 mL). Then, the mixturewas stirred at 0 DC for 3hr until TLC and LCMS analysis showed that most of 2-bromo-5-nitroaniline was consumed. SnCh (1.90 g, 10 mmol) in cone. HCl (3 mL) was slowly added. Themixture was then stirred at RT for 2 hr before re-cooled to 0 DC. Then, the PH was adjusted withsat. aq. NaHC03 to 7-8. The mixture was extracted with ethyl acetate (3 x 50 mL). The combined organic layers were washed with brine, dried over Na2S04, filtered and concentratedto get crude product which was further chromatographed on 10 g of silica gel using PE/EA (2011to 411) as eluant to afford 560 mg (51%) of (2-bromo-5-nitrophenyl)hydrazine as a orange solid.1H NMR (400 MHz, DMSO-d6) 8 7.89 (d, J = 2.8 Hz, IH), 7.60 (d, J = 8.6 Hz, IH), 7.29 (dd, J= 2.8, 8.6 Hz, IH), 7.04 (s, IH), 4.38 (s, 2H). MS (ESI) m/e [M+ It 232, 234.
References:
WO2014/173289,2014,A1 Location in patent:Paragraph 0465; 0467; 0468