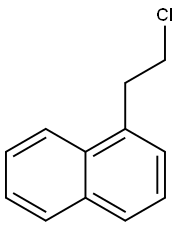
1-(2-CHLOROETHYL)NAPHTHALENE synthesis
- Product Name:1-(2-CHLOROETHYL)NAPHTHALENE
- CAS Number:41332-02-9
- Molecular formula:C12H11Cl
- Molecular Weight:190.67
Yield: 29%
Reaction Conditions:
with 4-methoxypyridine;potassium phosphate;4,4'-di-tert-butyl-2,2'-bipyridine;nickel dibromide in 1,2-dimethoxyethane at 70; for 24 h;Sealed tube;Inert atmosphere;chemoselective reaction;Suzuki Coupling;
Steps:
Ni-catalyzed Suzuki-type chloroethylation using ClCH2CH2Br as electrophile
General procedure: To a 35 mL sealed tube was added K3PO4 (127.4 mg, 0.6 mmol), NiBr2 (4.4 mg, 0.02 mmol), dtbpy (5.4 mg, 0.02 mmol), 4-MeO-Py (6.5 mg, 0.06 mmol), followed by a mixture of arylboronic acid 1 (0.30 mmol) and ClCH2CH2Br 2b (28.7 mg, 17.0 μL, 0.20 mmol) in DME (1.0 mL) under nitrogen. The sealed tube was screw capped. The reaction mixture was stirred at 70 °C (oil bath) for 24 h, cooled to room temperature, poured into saturated aqueous ammonium chloride solution and extracted with Et2O (10*3 mL). The organic phase was combined, dried over anhydrous Na2SO4, concentrated and further purified by silica gel column chromatography to give the products.
References:
Yang, Yi;Cai, Junjie;Luo, Gen;Tong, Xia;Su, Yumei;Jiang, Yan;Liu, Yingle;Zheng, Yubin;Zeng, Jijiao;Li, Chaolin [Tetrahedron Letters,2019,vol. 60,# 16,p. 1130 - 1134] Location in patent:supporting information
773-99-9
193 suppliers
$10.00/1g

41332-02-9
53 suppliers
$46.00/1g
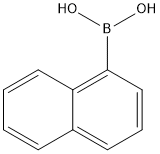
13922-41-3
485 suppliers
$5.00/1g

107-06-2
442 suppliers
$14.00/25g

41332-02-9
53 suppliers
$46.00/1g
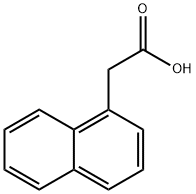
86-87-3
564 suppliers
$13.50/25G

41332-02-9
53 suppliers
$46.00/1g