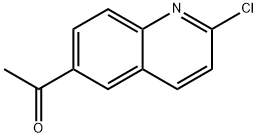
1-(2-chloroquinolin-6-yl)ethanone synthesis
- Product Name:1-(2-chloroquinolin-6-yl)ethanone
- CAS Number:1253972-47-2
- Molecular formula:C11H8ClNO
- Molecular Weight:205.64
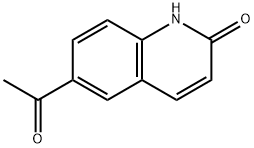
1253972-46-1
4 suppliers
inquiry

1253972-47-2
8 suppliers
inquiry
Yield:1253972-47-2 60%
Reaction Conditions:
Stage #1: 6-acetylquinolin-2(1H)-onewith trichlorophosphate for 1 h;Reflux;
Stage #2: with sodium hydrogencarbonate
Steps:
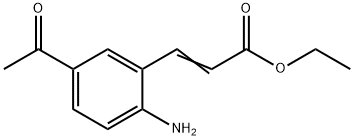
4-Aminoacetophenone was converted to l-(4-amino-3-iodophenyl)ethanone according to a procedure reported in US 5,874,430Al. l-(4-Amino-3-iodo-phenyl)ethanone (1.0 eq) was dissolved in toluene (5 vol) to which potassium acetate (1.2 eq), ethyl acrylate (1.2 eq) and triphenylphosphine (0.5 eq) were added. The mixture was degassed with argon for 20 min after which Pd(OAc)2 (0.25 eq) was added. The reaction mixture was heated at 1000C overnight. The reaction mass was filtered through celite and the filtrate washed with water and extracted with EtOAc. The EtOAc layer was washed with brine and dried over sodium sulfate. The EtOAc layer was evaporated under vacuum. Purification of the residue by column chromatography (silica gel, EtOAc:hexane 1.5:8.5) afforded 50% of 3- (5-acetyl-2-aminophenyl)acrylic acid ethyl ester. 1H-NMR (400 MHz, DMSO-d6) δ 8.03(s, IH), 7.84-7.88 (d, IH, J= 16.0 Hz), 7.64-7.66 (d, IH, J=8.0 Hz), 6.69-6.71 (d, IH, J=8.8 Hz), 6.47-6.52 (s, IH), 6.47 (s, 2H), 4.15-4.20 (m, 2H), 2.44 (s, 3H), 1.23-1.27 (t, 3H, J=7.2Hz) ppm.
References:
WO2010/127208,2010,A1 Location in patent:Page/Page column 114-115
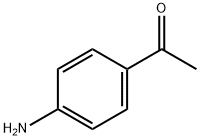
99-92-3
521 suppliers
$6.00/10g

1253972-47-2
8 suppliers
inquiry
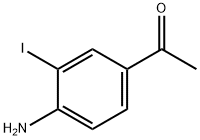
97776-06-2
28 suppliers
inquiry

1253972-47-2
8 suppliers
inquiry
1253972-45-0
0 suppliers
inquiry

1253972-47-2
8 suppliers
inquiry