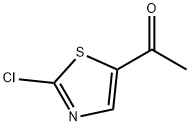
1-(2-chlorothiazol-5-yl)ethanone synthesis
- Product Name:1-(2-chlorothiazol-5-yl)ethanone
- CAS Number:885229-41-4
- Molecular formula:C5H4ClNOS
- Molecular Weight:161.61
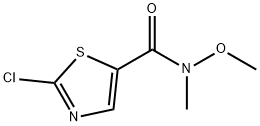
1234014-27-7
4 suppliers
inquiry
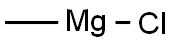
676-58-4
294 suppliers
$15.00/25ml

885229-41-4
33 suppliers
$38.00/250mg
Yield:885229-41-4 80%
Reaction Conditions:
Stage #1: 2-chloro-N-methoxy-N-methyl-1,3-thiazole-5-carboxamide;methylmagnesium chloride in tetrahydrofuran at -10 - 20;Inert atmosphere;
Stage #2: with ammonium chloride in tetrahydrofuran;
Steps:
A
A 4-neck 12 L round bottom flask equipped with a nitrogen inlet, mechanical stirrer and thermowell was charged with 2- chloro-N-methoxy-N-methylthiazole-5-carboxamide A.2 (157 g, 0.762 mol) and anhydrous THF (3.14 L). The resulting mixture was cooled to -10 °C by ice/salt bath and methyl magnesium chloride (3 M solution in THF, 305 mol, 0.914 mol) was added dropwise to maintain the temperature below 0 °C. After addition, the cooling bath was removed and the reaction mixture was stirred at room temperature overnight. The reaction was quenched by the slow addition of saturated ammonium chloride solution and extracted with MTBE (2 x 4 L). The organic layers were combined, washed with brine (2 L) and dried over sodium sulfate. The solvent was evaporated in vacuo to afford a crude solid, which was further purified by flash chromatography on silica gel (MTBE/hexanes as elute) to give l-(2-chlorothiazol-5-yl)ethanone A.3 as a white solid (135 g, 80% yield).
References:
WO2010/78408,2010,A1 Location in patent:Page/Page column 74; 75
53159-71-0
63 suppliers
$45.00/10mg

885229-41-4
33 suppliers
$38.00/250mg
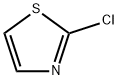
3034-52-4
224 suppliers
$12.00/5g
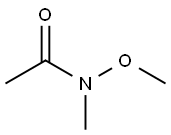
78191-00-1
287 suppliers
$6.00/5g

885229-41-4
33 suppliers
$38.00/250mg
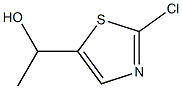
40982-18-1
2 suppliers
inquiry

885229-41-4
33 suppliers
$38.00/250mg