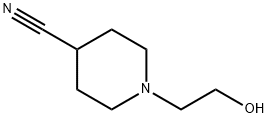
1-(2-Hydroxyethyl)piperidine-4-carbonitrile synthesis
- Product Name:1-(2-Hydroxyethyl)piperidine-4-carbonitrile
- CAS Number:21168-73-0
- Molecular formula:C8H14N2O
- Molecular Weight:154.21
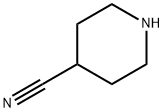
4395-98-6

540-51-2

21168-73-0
General procedure for the synthesis of 1-(2-hydroxyethyl)piperidine-4-carbonitrile from 4-cyanopiperidine and 2-bromoethanol: 2-bromoethanol (1.36 g, 11 mmol) and piperidine-4-carbonitrile (1.0 g, 9.1 mmol) were dissolved in 5 mL of dimethylformamide. Subsequently, 4.0 g of anhydrous sodium carbonate and 0.08 g of sodium iodide were added to the resulting solution and the mixture was stirred at 130 °C for 4 hours. Upon completion of the reaction, the mixture was poured into ice water and extracted with ethyl acetate. The organic phase was purified by silica gel column chromatography to afford 1-(2-hydroxyethyl)piperidine-4-carbonitrile (300 mg, 18% yield).1H-NMR (CDCl3, 400 MHz): δ 3.58 (t, J = 4.6 Hz, 2H), 2.60-2.75 (m, 4H), 2.50-2.60 (m, 2H), 2.31-2.46 (m, 2H). 2H), 1.70-2.00 (m, 4H).
Yield:21168-73-0 18%
Reaction Conditions:
with sodium carbonate;sodium iodide in N,N-dimethyl-formamide at 130; for 4 h;
Steps:
1.27
27. 2-(4-(((3-(3-(Trifluoromethoxy)Dhen vDimidazof 1.2-blpyridazin-6- yl)amino)methyl)DiDeridin-1-yl)ethanol EX. 8-27). [00347] Bromoethanol (1 .36 g, 1 1 mmol) and pipehdine-4-carbonithle (1 .0 g, 9.1 mmol) 5 ml_ of dimethylformamide. After this, 4.0 g of anhydrous sodium carbonate and 0.08 g of sodium iodide were added to the resulting solution, and the mixture was stirred for 4 hours at 130 °C. Then the mixture was poured into ice water and extracted with ethyl acetate. The oily extract obtained was purified by silica gel chromatography 1 -(2-hydroxyethyl)piperidine-4-carbonitrile (300 mg, 18%). [00348] 1 H-NMR (CDCI3/400 MHz): δ 3.58 (t, J = 4.6 Hz, 2H), 2.60 - 2.75 (m, 4 H), 2.50 - 2.60 (m, 2H), 2.31 -2.46 (m, 2H), 1 .70 - 2.00 ( m, 4 H).
References:
WO2013/13188,2013,A1 Location in patent:Paragraph 00347; 00348