1-(2-methoxy-4-nitrophenyl)-4-methyl-1H-imidazole synthesis
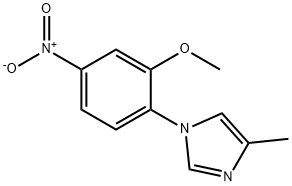
- Product Name:1-(2-methoxy-4-nitrophenyl)-4-methyl-1H-imidazole
- CAS Number:958245-17-5
- Molecular formula:C11H11N3O3
- Molecular Weight:233.22
Yield:958245-17-5 85%
Reaction Conditions:
with potassium hydroxide in dimethyl sulfoxide at 80; for 8 h;Inert atmosphere;
Steps:
7 Synthesis of l-(2-Methoxy-4-nitrophenyl)-4-methyl-lH-imidazole
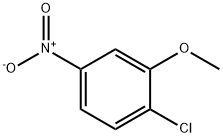
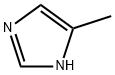
Synthesis of l-(2-Methoxy-4-nitrophenyl)-4-methyl-lH-imidazole [0304] To a stirred solution of 2-chloro-5-nitroanisole (3 g, 16.0 mmol) in DMSO (15 mL) under argon atmosphere were added 4-methyl imidazole (5.2 g, 64.0 mmol), and KOH (1.34 g, 24.0 mmol) at RT. The reaction mixture was stirred at 80 °C for 8 h. After the consumption of the starting materials (monitored by TLC), the reaction was diluted with water (50 mL) to afford the solid which was filtered and dried in vacuo to afford l-(2- Methoxy-4-nitrophenyl)-4-methyl-lH-imidazole (3.2 g, 85%) as a yellow solid. 1H-NMR (DMSO-de, 400 MHz): δ 7.98-7.95 (m, 3H), 7.72-7.67 (m, 1H), 7.28 (s, 1H), 3.98 (s, 3H), 2.16 (s, 3H); TLC: 100% EtOAc (R 0.3).
References:
WO2015/66697,2015,A1 Location in patent:Paragraph 0304
822-36-6
492 suppliers
$9.00/1g
454-16-0
161 suppliers
$10.00/1g

958245-17-5
22 suppliers
inquiry

1255701-91-7
0 suppliers
inquiry