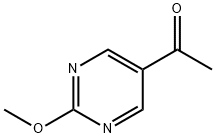
1-(2-Methoxypyrimidin-5-yl)ethanone synthesis
- Product Name:1-(2-Methoxypyrimidin-5-yl)ethanone
- CAS Number:1056174-56-1
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
931-63-5

75-36-5

1056174-56-1
GENERAL METHODS: 2-methoxypyrimidine (1c, 0.216 mg, 2 mmol) was mixed with acetyl chloride (2a, 0.157 mg, 2 mmol), and CTAB or CTAC (0.001 mol) was added as a catalyst and dissolved in dichloroethane. The reaction mixture was heated in a microwave synthesizer (Biotage Initiator+ SP Wave model, power 0.200 W, frequency 2.45 GHz, capped at 60 W at steady state) for 5 min, and the reaction conditions were controlled to be at 100 °C and 2 bar pressure. Upon completion of the reaction, the target product 1-(2-methoxypyrimidin-5-yl)ethanone (3o) was isolated and purified by column chromatography using silica gel as stationary phase and mixed solvent of ethyl acetate-petroleum ether as eluent.

931-63-5
60 suppliers
$253.05/1G

75-36-5
589 suppliers
$17.92/100G

1056174-56-1
20 suppliers
inquiry
Yield:1056174-56-1 97%
Reaction Conditions:
with cetyltrimethylammonim bromide;cetyltrimethylammonium chloride in 1,2-dichloro-ethane at 70; for 0.0833333 h;Microwave irradiation;Micellar solution;Friedel-Crafts Acylation;
Steps:
General procedure for microwave-assisted synthesis
General procedure: The mixture of anisole(1c, 0.216 mg, 2 mmol) and acetyl chloride (2a, 0.157 mg, 2 mmol) was treated with CTAB/CTAC, (0.001 mol), in dichloroethane and the resulting reaction mixture was heated in a controlled microwave synthesizer (BiotageInitiator+SP Wave model (0.200W at 2.45 GHz, capped at 60W duringsteady state) for 5 min (attains temperature 100 C and 2 bar pressure). The final product (3o) was isolated by absorbing the reaction mixture into silica geland purifying it by column chromatography using ethyl acetate-petroleumether as the eluent.
References:
Rajendar Reddy;Rajanna;Uppalaiah [Tetrahedron Letters,2013,vol. 54,# 26,p. 3431 - 3436]