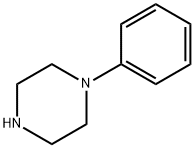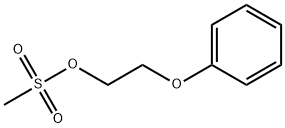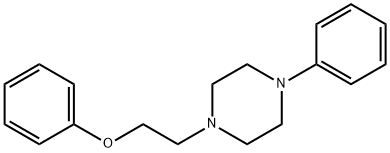
1-(2-phenoxyethyl)-4-phenyl-piperazine synthesis
- Product Name:1-(2-phenoxyethyl)-4-phenyl-piperazine
- CAS Number:1037-20-3
- Molecular formula:C18H22N2O
- Molecular Weight:282.38
Yield:1037-20-3 97%
Reaction Conditions:
with lithium carbonate in acetonitrile; for 24 h;Reflux;Inert atmosphere;
Steps:
4.1.7. General procedure for preparation of functionalized N-phenylpiperazine derivatives (7a-b), (7d-k), (8a-b) and (8d-f)
General procedure: To a suspension of corresponding mesylate derivative (13A-B), (16A-B), (18A-B), (20A-B), (24A-B) (0.50 mmol) and lithium carbonate (0.07 g, 1.00 mmol) in acetonitrile (6 mL), was added the adequate N-phenylpiperazine derivative (2.00 mmol). The mixture was kept at reflux for 24 h under vigorous agitation and nitrogen atmosphere. At the end of this time the solution was concentrated in a rotatory evaporator, solubilized in dichloromethane and mixed with silica gel. The material was separated by chromatography on silica gel eluted with dichloromethane, followed by chloroform to give the desired N-phenylpiperazine derivatives of 1,3-benzodioxolyl (7) and phenyl (8) series, as described next.
References:
Romeiro, Luiz A.S.;Da Silva Ferreira, Marcos;Da Silva, Leandro L.;Castro, Helena C.;Miranda, Ana L.P.;Silva, Cláudia L.M.;No?l, Franois;Nascimento, Jéssica B.;Araújo, Claudia V.;Tibiri?á, Eduardo;Barreiro, Eliezer J.;Fraga, Carlos A.M. [European Journal of Medicinal Chemistry,2011,vol. 46,# 7,p. 3000 - 3012] Location in patent:experimental part
66003-76-7
155 suppliers
$6.00/250mg

1037-20-3
7 suppliers
inquiry
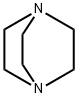
280-57-9
598 suppliers
$5.00/5g

1037-20-3
7 suppliers
inquiry

144930-50-7
14 suppliers
inquiry

1037-20-3
7 suppliers
inquiry
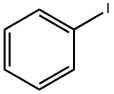
591-50-4
500 suppliers
$10.00/1g

1037-20-3
7 suppliers
inquiry