
1(2H)-Isoquinolinone, 4-bromo-7-methyl- synthesis
- Product Name:1(2H)-Isoquinolinone, 4-bromo-7-methyl-
- CAS Number:877263-69-9
- Molecular formula:C10H8BrNO
- Molecular Weight:238.08
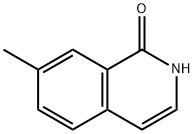
26829-47-0
49 suppliers
$394.89/1g

877263-69-9
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with bromine;acetic acid at 20; for 4 h;
Steps:
41.B
Step B: A solution of bromine (4.8 ml, 94 mmol) in acetic acid (50 ml) was added dropwise to a solution of the product from Step A (15.0 g, 94 mmol) in acetic acid (300 ml) at room temperature. The mixture was stirred at room temperature for 4 hours, poured into iced water, extracted with dichloromethane three times and dried over magnesium sulfate to give the desired product (24.2 g): 1H NMR (300 MHz, CDCl3) δ 8.24 (s, 1H), 7.80 (d, J=8.4 Hz, 1H), 7.63 (dd, J=8.4, 1.8 Hz, 1H), 7.39 (s, 1H), 2.55 (s, 3H).
References:
US2006/52378,2006,A1 Location in patent:Page/Page column 104