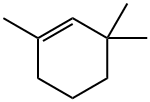
1,3,3-trimethylcyclohexene synthesis
- Product Name:1,3,3-trimethylcyclohexene
- CAS Number:503-47-9
- Molecular formula:C9H16
- Molecular Weight:124.22
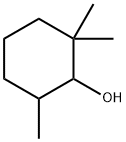
10130-91-3
0 suppliers
inquiry

503-47-9
2 suppliers
inquiry
Yield:503-47-9 96.8 %
Reaction Conditions:
with toluene-4-sulfonic acid at 60 - 65;Reagent/catalyst;Temperature;Time;
Steps:
4-6 Example 4: Preparation of 1,3,3-trimethylcyclohexene (VI) (Route 2)
In the 500 milliliter stainless steel autoclave that is connected with stirring, thermometer, add 300 gram acetonitrile, 70.1 gram (0.5 mole) 2,2,6-trimethylcyclohexanone (IV), 0.8 gram palladium mass content is 5% Palladium-carbon catalyst, nitrogen replacement for three times, and then hydrogen gas was introduced, keeping the hydrogen pressure at 0.8-1.0 MPa, and reacting at 40-45°C for 4 hours.After cooling down to room temperature, replace with nitrogen three times, open the kettle, remove the catalyst by filtration, transfer the filtrate of the gained 2,2,6-trimethylcyclohexanol (V) to a 500 milliliter IV tank connected with stirring, thermometer and reflux condenser. Add 1.0 g of p-toluenesulfonic acid to the flask, stir at 60-65°C for 4 hours, cool to 20-25°C, add 100 g of dichloromethane, separate layers, and extract the water layer twice with dichloromethane, each 50 grams at a time, the organic phases were combined, dichloromethane and acetonitrile were recovered by atmospheric distillation, and further distillation (receiving fractions at 115-130 ° C) gave 60.1 grams of colorless transparent liquid 1,3,3-trimethylcyclohexene (VI) , The gas phase purity is 99.6%, and the yield is 96.8% based on 2,2,6-trimethylcyclohexanone.
References:
CN115677465,2023,A Location in patent:Paragraph 0102-0110
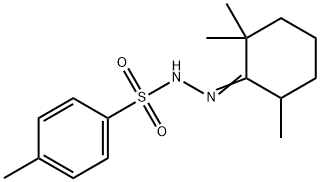
41780-73-8
0 suppliers
inquiry

503-47-9
2 suppliers
inquiry

37779-25-2
0 suppliers
inquiry

503-47-9
2 suppliers
inquiry
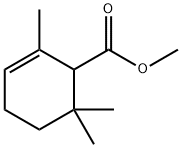
28043-10-9
21 suppliers
inquiry

503-47-9
2 suppliers
inquiry

