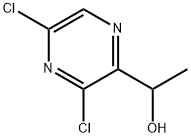
1-(3,5-Dichloropyrazin-2-yl)ethanol synthesis
- Product Name:1-(3,5-Dichloropyrazin-2-yl)ethanol
- CAS Number:136866-33-6
- Molecular formula:C6H6Cl2N2O
- Molecular Weight:193.03

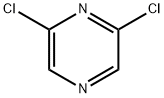
75-16-1
276 suppliers
$12.00/10ml
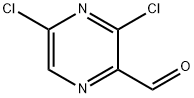
136866-27-8
87 suppliers
$106.04/50mgs:

136866-33-6
4 suppliers
inquiry
Yield:136866-33-6 96%
Reaction Conditions:
in tetrahydrofuran at 5; for 0.166667 h;Inert atmosphere;
Steps:
10.i (i) 1-(3,5-Dichloro-pyrazin-2-yl)-ethanol
3,5-Dichloro-pyrazine-2-carbaldehyde (5.0 g) was dissolved in dry tetrahydrofuran (100 ml) in a reaction vessel equipped with a magnetic stirring bar under an argon atmosphere. The solution was cooled on an ice-bath before slow addition of 10.3 ml methylmagnesium bromide solution (3M in tetrahydrofuran), keeping the internal temperature in the reaction vessel below 5°C. After the addition the cooling bath was removed and the reaction mixture stirred for another 10 min. Then the reaction mixture was quenched with a saturated aqueous sodium bicarbonate solution (100 ml) and extracted with EtOAc (3 x 200 ml). The combined aqueous phases were dried over sodium sulfate, filtered and evaporated to afford 1-(3,5-dichloro-pyrazin-2-yl)-ethanol as a dark brown oil. Yield: 5.23g (96%).
References:
EP2570415,2013,A1 Location in patent:Paragraph 0130