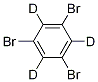
1,3,5-Tribromobenzene-d3 synthesis
- Product Name:1,3,5-Tribromobenzene-d3
- CAS Number:52921-77-4
- Molecular formula:C6Br3D3
- Molecular Weight:317.8185
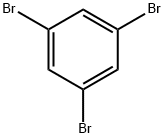
626-39-1
484 suppliers
$5.00/5g

52921-77-4
15 suppliers
inquiry
Yield:52921-77-4 97.1%
Reaction Conditions:
with water-d2;silver(l) oxide;2-(dicyclohexylphosphino)-2'-methylbiphenyl in toluene at 60 - 80;Reagent/catalyst;
Steps:
4.S41-4.S.45
S41) Obtain each component polyhalogen aromatic compound, silver salt catalyst, phosphine ligand, deuterium water and toluene solvent, wherein said polyhalogen aromatic compound: said silver salt catalyst: said phosphine ligand: said The molar ratio of deuterium water: the toluene solvent is 1:(0.05-0.1):(0.05-0.3):(20-40):(5-50). The molecular structural formula of the polyhalogen aromatic compound is: Wherein, the R is hydrogen, ester group, cyano group, carbonyl group, hydroxyl group, alkoxy group, alkyl group, amide group, amino group, borate, alkynyl group, alkenyl group, phenyl group, fluorine group, heterocyclic aryl group The X is any one of bromine, chlorine, and iodine; the n is any one of 2, 3, 4, and 5. In the embodiment of the present application, the polyhalogen aromatic compound is 1,3,5-tribromobenzene. The phosphine ligand is 2-dicyclohexylphosphorus-2'-methylbiphenyl, 2-dicyclohexylphosphino-2'-(N,N-dimethylamine)-biphenyltriphenylphosphine, two Phenylcyclohexylphosphine, 2-(dicyclohexylphosphine)biphenyl, 2-dicyclohexylphosphine-2,4,6-triisopropylbiphenyl, 2-di-tert-butylphosphine-2'-methyl Any of phosphine ligands such as biphenyl, triphenylphosphine, tricyclohexylphosphine, tris(2-furyl)phosphine, 1,2-bis(diphenylphosphine)ethane, etc. The silver salt catalyst is any one of silver carbonate, silver oxide, silver chloride, and silver bromide. S42) Add the silver salt catalyst, the deuterium water, the polyhalogen aromatic compound and the toluene solvent into the reactor to obtain a mixed solution. Specifically, into the 10 mL reactor, add 0.05 mmol of the silver salt catalyst, 0.05 mmol of phosphine ligand, 0.44 g, 22 mmol of deuterium water, 1 mmol of 1,3,5-tribromobenzene and 22 mmol The toluene solvent. In the embodiment of the present application, the reaction liquid includes but is not limited to a reaction flask. S43) Heat treatment of the mixed solution. Specifically, the mixed solution is heated at a temperature of 60-80° C. for 11-13 hours to make the mixed solution fully react. S44) After the reaction of the mixed solution is complete, add saturated ammonium chloride solution to the reactor for quenching. Specifically, 99-101 ml of the ammonium chloride solution, preferably 100 ml of the ammonium chloride solution is added to the reactor, so that the mixed solution in the reactor undergoes a quenching reaction. S45) Adding a dichloromethane solvent to the reactor, and after extraction, combining the organic phases, and rotary evaporation and concentration, a deuterated polyhalogen aromatic compound is obtained. Specifically, 99-101 ml of the dichloromethane solvent, preferably 100 ml of the dichloromethane solvent is added to the reactor. Wherein, the dichloromethane solvent can be used to extract three to four times, and the organic phases extracted several times can be combined into a 250ml eggplant-shaped bottle, using a rotary evaporator, the rotating speed is 120rpm, the temperature is 37°C, and the vacuum degree is 0.1Mpa , Treat for 3min, get deuterated polyhalogen aromatic compound, namely deuterated 1,3,5-tribromobenzene. Among them, the deuterated 1,3,5-tribromobenzene is 305 mg, the yield is 97.1%, the deuteration rate is 94%, and the analytical purity is 99%.
References:
CN113149801,2021,A Location in patent:Paragraph 0096-0112