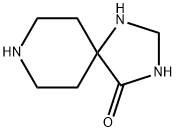
1,3,8-Triaza-spiro[4.5]decan-4-one synthesis
- Product Name:1,3,8-Triaza-spiro[4.5]decan-4-one
- CAS Number:56186-25-5
- Molecular formula:C7H13N3O
- Molecular Weight:155.2
![1-PHENYL-1,3,8-TRIAZASPIRO[4.5]DECAN-4-ONE](/CAS/GIF/1021-25-6.gif)
1021-25-6
115 suppliers
$39.00/100mg
![1,3,8-Triaza-spiro[4.5]decan-4-one](/CAS/20180703/GIF/56186-25-5.gif)
56186-25-5
13 suppliers
inquiry
Yield:56186-25-5 152 mg (55%)
Reaction Conditions:
with potassium carbonate in pyridine;hexane;dichloromethane;ethyl acetate;
Steps:
12.4 Step 4
Step 4 A solution of XIV (237 mg, 0.659 mmol), 1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one (458 mg, 1.98 mmol), and powdered, anhydrous potassium carbonate (456 mg, 3.30 mmol) in dry pyridine (7 mL) was heated at 75° C. under nitrogen overnight. The black mixture was diluted with dichloromethane (1 vol) and filtered through Celite, washing the black sludge well with dichloromethane. The filtrate was concentrated in vacuo (a slight amount of pyridine was allowed to remain), and the residue was taken-up in a large volume of dichloromethane and chromatographed on 20 g of 230-400 silica gel using 75% ethyl acetate/hexane to give 152 mg (55%) of XIm as a yellowed solid; for analysis, recrystallization from ethyl acetate/ethanol/hexane gave a pale yellow-tan solid, mp 228-230 (dec): Rf 0.17 (75% ethyl acetate/hexane); 2955, 2925, 2854, 1711, 1511, 1497, 1456, 1360, 1236, 1094 cm-1; 1 H NMR (300 MHz, DMSO-d6) 10.93 (broad s, 1H, indole N--H), 8.65 (broad s, 1H, lactam N--H), 7.25 (t, J=7.7 Hz, 2 H, phenyl meta H's), 7.19 (t, J=2.7 Hz, 1H, vinylic H), 6.86 (m, 3 H, phenyl ortho H's and aromatic H), 6.76 (t, J=7.3 Hz, 1H, phenyl para H), 6.65 (d, J=8.6 Hz, 1H, aromatic H), 6.31 (m, 1H, vinylic H), 4.58 (s, 2 H, N--CH2 --N), 4.43 (m, 1 H, O--CH), 4.34 (m, 1H, O--CH2 a), 4.02 (dd, J=11.3 Hz, J=6.7 Hz, 1H, O--CH2b), 3.0-2.5 (m, 8H, N--CH2 's and N--C--CH2 a's), 1.57 (m, 2 H, N--C--CH2 b's); 1 3C NMR (75.5 MHz, DMSO-d6) 176.2, 143.3, 134.6, 134.4, 132.3, 129.0, 124.6, 118.4, 117.6, 114.2, 112.0, 104.0, 97.4, 71.4, 66.3, 58.7, 58.0, 57.8, 50.7, 49.6, 28.5; HRMS, m/e 418.2015 (C2 4H2 6N4 O3 requires 418.2005); Anal. Calcd for C2 4H2 6N4 O3: C, 68.88; H, 6.26; N, 13.39. Found: C, 68.60; H, 6.19; N, 13.39.
References:
US5302599,1994,A
![8-BENZYL-1,3,8-TRIAZA-SPIRO[4.5]DEC-1-EN-4-ONE](/CAS/GIF/1017789-30-8.gif)
1017789-30-8
14 suppliers
inquiry
![1,3,8-Triaza-spiro[4.5]decan-4-one](/CAS/20180703/GIF/56186-25-5.gif)
56186-25-5
13 suppliers
inquiry
![8-Benzyl-1,3,8-triaza-spiro[4.5]decan-4-one](/CAS/GIF/170921-48-9.gif)
170921-48-9
39 suppliers
$41.00/100mg
![1,3,8-Triaza-spiro[4.5]decan-4-one](/CAS/20180703/GIF/56186-25-5.gif)
56186-25-5
13 suppliers
inquiry
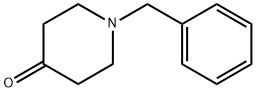
3612-20-2
446 suppliers
$6.00/10g
![1,3,8-Triaza-spiro[4.5]decan-4-one](/CAS/20180703/GIF/56186-25-5.gif)
56186-25-5
13 suppliers
inquiry
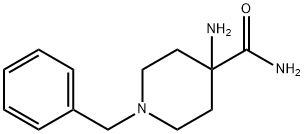
170921-49-0
68 suppliers
$45.00/50mg
![1,3,8-Triaza-spiro[4.5]decan-4-one](/CAS/20180703/GIF/56186-25-5.gif)
56186-25-5
13 suppliers
inquiry