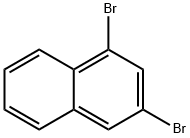
1,3-Dibromonaphthalene synthesis
- Product Name:1,3-Dibromonaphthalene
- CAS Number:52358-73-3
- Molecular formula:C10H6Br2
- Molecular Weight:285.96
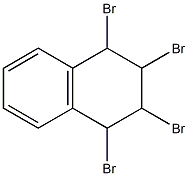
19922-78-2
0 suppliers
inquiry

52358-73-3
65 suppliers
inquiry
Yield:52358-73-3 88%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran at 20;
Steps:
1,3-dibromonaphthalene 5
1,2,3,4-tetrabromo-1,2,3,4-tetrahydronaphthalene (3.0 g, 6.7 mmol, 1 eq.) in 27 ml of dry THF was added to the suspension of potassium tert-butoxide (1.9 g, 16.7 mml, 2.5 eq.) in dry THF (8 ml). The reaction mixture was stirred at r.t. overnight. Next day water was added to the mixture and the mixture was extracted with diethyl ether three times, dried over MgSO4 and evaporated to dryness. The residue was filtered through a short silica column using light petroleum (bp = 40 - 60°C) as an eluent. Product (1.69 g, 88 %) was obtained as a colourless oil that solidified upon freezing (-20°C). The spectroscopic data were consistent with that previously reported.1, 2 1H NMR (400 MHz, CD2Cl2) δ 8.20 (d, J = 8.3 Hz, 1H, H-8), 8.02 (d, J = 1.9 Hz, 1H, H-4), 7.91 (d, J = 1.9 Hz, 1H, H-2), 7.79 (dd, J = 8.2, 1.5 Hz, 1H, H-5), 7.66 - 7.56 (m, 2H, H-6,7). 13C NMR (101 MHz, CD2Cl2) δ 135.7 (C-4a), 133.1 (C-2), 131.2 (C-8a), 130.6 (C-4), 128.4 (C-7), 128.4 (C-6), 128.1 (C-5), 127.6 (C-8), 123.9 (C-1), 119.3 (C-3).
References:
Turunen, Lotta;Németh, Flóra Boróka;Decato, Daniel A.;Pápai, Imre;Berryman, Orion B.;Erdélyi, Máté [Bulletin of the Chemical Society of Japan,2021,vol. 94,# 1,p. 191 - 196] Location in patent:supporting information
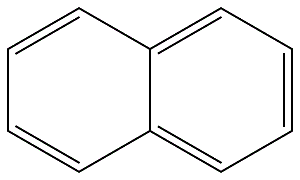
91-20-3
530 suppliers
$13.19/250mg

52358-73-3
65 suppliers
inquiry
20191-76-8
77 suppliers
$15.00/250mg

52358-73-3
65 suppliers
inquiry
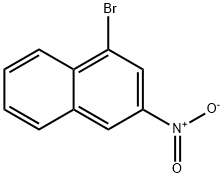
7499-65-2
16 suppliers
inquiry

52358-73-3
65 suppliers
inquiry
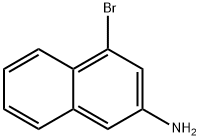
74924-94-0
69 suppliers
inquiry

52358-73-3
65 suppliers
inquiry