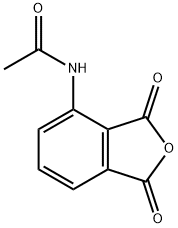
1,3-Dioxo-2-isoindolineaceticacid synthesis
- Product Name:1,3-Dioxo-2-isoindolineaceticacid
- CAS Number:6296-53-3
- Molecular formula:C10H7NO4
- Molecular Weight:205.17
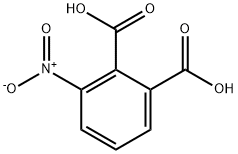
603-11-2

108-24-7

6296-53-3
General procedure for the synthesis of N-(1,3-dioxo-1,3-dihydroisobenzofuran-4-yl)acetamide from 3-nitrophthalic acid and acetic anhydride: 3-nitrophthalic acid (100.0 g) and acetic anhydride (1 L) were added to a hydrogenation kettle and heated to 80 °C until the raw materials were completely dissolved. Subsequently, the reaction mixture was cooled to room temperature and 10% Pd/C catalyst (3 g) was added. The atmosphere in the reaction kettle was replaced twice with nitrogen, then hydrogen was introduced to a pressure of 1 MPa, and the reaction was kept at room temperature until hydrogen absorption stopped. Upon completion of the reaction, the hydrogen was vented and the reactor was purged with nitrogen. Next, the reaction mixture was heated to 110-115 °C until the feedstock or intermediate completely disappeared. Thermal filtration was carried out and the filtrate was cooled to 0-5 °C, the product was collected by filtration and washed with glacial acetic acid. A final light yellow crystalline product of 80.6 g was obtained with 83% yield and 99.0% HPLC purity.

603-11-2
646 suppliers
$15.00/25 g

108-24-7
0 suppliers
$14.00/250ML

6296-53-3
297 suppliers
$6.00/250mg
Yield:6296-53-3 83%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen at 80 - 115; under 7500.75 Torr;Autoclave;Temperature;
Steps:
6 Preparation of 3-acetylamino phthalic anhydride
2-nitrophthalic acid (100. 0 g), acetic anhydride (1 L) was added to a hydrogenation kettle, heated to 80 ° C,After the raw material disappeared, the mixture was cooled to room temperature, 10% Pd / C (3 g) was added, replaced with nitrogen twice, hydrogen was introduced to IMPa,The reaction is maintained at room temperature until no hydrogen is absorbed, vented, blown with nitrogen,Heated to 110-115 ° C reaction to the raw material or intermediate state disappeared,Hot filter, cooled to 0-5 ° C, filtered, washed with glacial acetic acid to give pale yellow crystals, dried 80. 6g, yield 83%, HPLC 99. 0%.
References:
Shanghai UTOpharm Company Limited;Luo, junzhi;Zeng, YuPing CN105294534, 2016, A Location in patent:Paragraph 0036; 0037
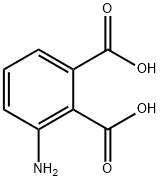
5434-20-8
250 suppliers
$41.00/1g

108-24-7
0 suppliers
$14.00/250ML

6296-53-3
297 suppliers
$6.00/250mg

5434-20-8
250 suppliers
$41.00/1g

6296-53-3
297 suppliers
$6.00/250mg

108-24-7
0 suppliers
$14.00/250ML
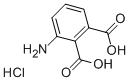
6946-22-1
186 suppliers
$33.10/1g

6296-53-3
297 suppliers
$6.00/250mg

603-11-2
646 suppliers
$15.00/25 g

6296-53-3
297 suppliers
$6.00/250mg