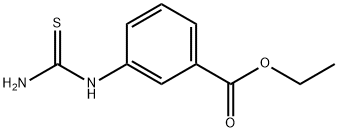
1-(3-ETHOXYCARBONYLPHENYL)-2-THIOUREA synthesis
- Product Name:1-(3-ETHOXYCARBONYLPHENYL)-2-THIOUREA
- CAS Number:20967-87-7
- Molecular formula:C10H12N2O2S
- Molecular Weight:224.28
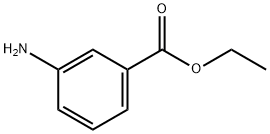
582-33-2
214 suppliers
$27.00/5g
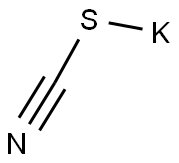
333-20-0
464 suppliers
$13.50/100G

20967-87-7
34 suppliers
$62.00/1g
Yield:20967-87-7 69%
Reaction Conditions:
Stage #1: 3-aminobenzoic acid ethyl esterwith sulfuric acid in chlorobenzene at -10; for 0.25 h;
Stage #2: potassium thioacyanatewith 18-crown-6 ether in chlorobenzene at 20 - 100; for 14.5 h;
Steps:
2
A solution of ethyl 3-aminobenzoate (90 mmol) in chlorobenzene (100 mL) was cooled to-10 °C and treated with sulfuric acid (45 mmol), dropwise. After 15 min, solid potassium thiocyanate (95 mmol) was added in several portions over 30 min followed by 18-crown-6 (250 mg). The mixture was heated at 100 °C for 10 h, allowed to cool to rt, and was maintained for an additional 4 h. The precipitated solids were isolated by filtration and were washed successively with chlorobenzene (25 mL) and hexanes (3 x 100 mL). The solid was suspended in water (300 mL) and the suspension was maintained 30 min. The product was isolated by filtration and washed with water (2 x 100 mL). The product was dried in a vacuum oven (55 °C) for 16 h, thus providing the thiocarbamate in 69% yield.'H NMR (500 MHz, Me2SO-d6) 5 1.32 (t, J= 7.5, 3H), 4.32 (q, J = 7, 2H), 7.44-7. 47 (m, 2H), 7.68-7. 76 (m, 3H), 8.05 (s, 1H), 9. 86 (s, 1H); MS (APCI) m/z 225 (M++1). A solution of thiocarbamate (12.2 mmol) in chloroform (10 mL) was added dropwise over a period of 40 min to a vigorously maintained mixture of ethyl 3- [(aminocarbonothioyl) amino] benzoate (5.78 mmol), glacial acetic acid (10 mL) and chloroform (10 mL). The mixture was maintained 30 min at rt and then was heated at 70 °C for 4 h. The mixture was allowed to cool to room temperature and maintained for an additional 13 h. The volatiles were removed under reduced pressure and the solid residue was suspended in a mixture of chloroform (10 mL) and acetone (10 mL). The product was isolated by filtration, washed successively. with acetone (5 mL) and hexanes (10 mL), and dried in a vacuum oven, thus providing the product in 95% yield as a mixture of ethyl 2-amino-1, 3-benzothiazole-7-carboxylate hydrobromide and ethyl 2-amino-1, 3- benzothiazole-5-carboxylate hydrobromide in a ratio of 95/5, respectively. This product was partitioned between saturated aqueous solution of sodium bicarbonate (25 mL) and a mixture of ethyl acetate (70 mL) and tetrahydrofuran (30 mL). The organic layer was separated, dried over anhydrous sodium sulfate and concentrated. The residue was crystallized form ethyl acetate, thus providing pure ethyl 2-amino-1, 3-benzothiazole-7- carboxylate. 1H NMR (500 MHz, Me2SO-d6) 8 1. 35 (t, J= 7.5, 3H), 4.36 (q, J= 7,2H), 7. 35 (t, J= 7. 5, 1H), 7.57 (d, J= 7, 1H), 7.61 (bs, 2H), 7.65 (d, J= 8, 1H) ; MS (EI) mlz 223 (M++1). iso-Amylnitrite (53 mmol) was added to a solution of ethyl 2-amino-1, 3-benzothiazole-7- carboxylate (5.40 g) in tetrahydrofuran (70 mL) and the mixture was heated at reflux for 4 h. The volatiles were removed under reduced pressure and the residue was purified by chromatography (0/100 to 5/95 methanol/dichloromethane), thus providing the ester in 71% yield. lH NMR (500 MHz, CDC13) 6 1. 47 (t, J = 7. 5,3H), 4.49 (q, J= 7, 2H), 7.62 (t, J= 8, 1H), 8.20 (d, J= 6.5, 1H), 8.33 (d, J= 8, 1H), 9.12 (s, 1H); MS (EI) m/z 208 (M++1). A 50% aqueous sodium hydroxide (10 mL) was added to a 0 °C solution of ethyl 1, 3-benzothiazole-7-carboxylate (16.89 mmol) in a mixture of methanol (65 mL), tetrahydrofuran (20 mL) and water (5 mL). The mixture was maintained at room temperature for 4 h and the volatiles were removed under reduced pressure. The residue was dissolved in water (100 mL) and concentrated hydrochloric acid was added to adjust the pH of the solution to 5. The mixture was cooled to 0 °C and maintained for 30 min. The product was isolated by filtration, washed with water (10 mL), and dried in vacuum oven (70 °C) for 16 h, thus providing the acid in 91% yield. 1H NMR (500 MHz, Me2 S O-d6) 8 7.71 (t, J = 7.5, 1H), 8.15 (d, J = 7, 1H), 8. 38 (d, J = 8, 1H), 9. 51 (s, 1H), 13.74 (bs, 1H); MS (APCI) m/z 178 (M-1).
References:
WO2005/92890,2005,A2 Location in patent:Page/Page column 71-73
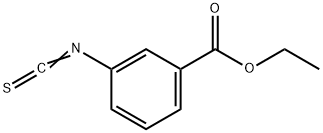
3137-84-6
33 suppliers
$60.00/100mg

20967-87-7
34 suppliers
$62.00/1g

582-33-2
214 suppliers
$27.00/5g

20967-87-7
34 suppliers
$62.00/1g