
1-(3-Fluorophenyl)-2,5-diMethylpyrrole synthesis
- Product Name:1-(3-Fluorophenyl)-2,5-diMethylpyrrole
- CAS Number:146135-21-9
- Molecular formula:C12H12FN
- Molecular Weight:189.23
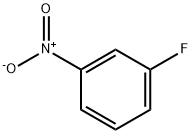
402-67-5
273 suppliers
$6.00/5g
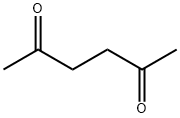
110-13-4
398 suppliers
$10.00/5g

146135-21-9
22 suppliers
$45.00/100mg
Yield:146135-21-9 87%
Reaction Conditions:
with indium;acetic acid in toluene at 80; for 10 h;Inert atmosphere;
Steps:
15 4.2.15. 1-(3-Fluorophenyl)-2,5-dimethyl-1H-pyrrole (17).14j
General procedure: Nitrobenzene derivative (1.0mmol) was added to a mixture of indium powder (460mg, 4.0mmol), and acetic acid (0.572mL, 10mmol) in toluene (2mL), followed by the addition of 2,5-hexadione or 1-phenyl-1,4-pentanedione (1.0mmol) in toluene (3mL). The reaction mixture was stirred at 80°C for 2,5-hexadione (or reflux for 1-phenyl-1,4-pentadione) under a nitrogen atmosphere. After the reaction was completed, the reaction mixture was diluted with ethyl acetate (30mL), filtered through Celite, poured into 10% NaHCO3 (30mL), and then extracted with ethyl acetate (30mL×3). The combined organic extracts were dried over MgSO4, filtered, and concentrated. The residue was eluted with hexane for most derivatives or ethyl acetate/hexane (v/v=5:95) for benzonitrile derivatives through a neutral silica gel column to give the corresponding pyrroles. The structures of the pyrroles were characterized by 1H NMR, 13C NMR, FTIR, and GC-MS, and were mostly known compounds. If it is an unknown compound, elemental analysis data were reported additionally.
References:
Lee, Hyunseung;Kim, Byeong Hyo [Tetrahedron,2013,vol. 69,# 32,p. 6698 - 6708]
625-86-5
205 suppliers
$20.00/25mL
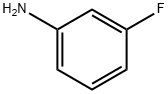
372-19-0
320 suppliers
$10.00/1g

146135-21-9
22 suppliers
$45.00/100mg

372-19-0
320 suppliers
$10.00/1g

110-13-4
398 suppliers
$10.00/5g

146135-21-9
22 suppliers
$45.00/100mg
