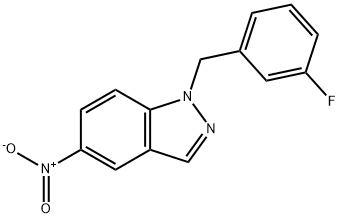
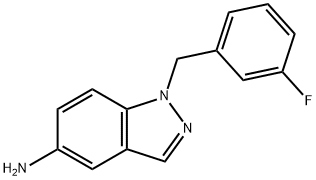
1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole synthesis
- Product Name:1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole
- CAS Number:529508-58-5
- Molecular formula:C14H10FN3O2
- Molecular Weight:271.25
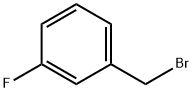
456-41-7
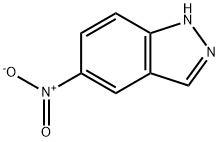
5401-94-5
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
General procedure for the synthesis of 1-[(3-fluorophenyl)methyl]-5-nitro-1H-indazole from 3-fluorobenzyl bromide and 5-nitroindazole: 5-nitroindazole (1 eq.), cesium carbonate (1.1 eq.), and DMF (5 v/v) were added to a reaction vessel. The mixture was heated to 70-80 °C and 3-fluorobenzyl bromide was added slowly and dropwise over 75 min. The progress of the reaction was monitored by HPLC until the area percentage (AP) of the nitroindazole with the combined isomers was less than 2%. After completion of the reaction, the mixture was cooled to 20 °C. The insoluble salt was removed by filtration and the filter cake was washed with DMF (2.7 v/v). Water (1.35 to 1.45 v/v) was added to the filtrate at 15-21 °C to induce crystallization of the product. The crystal slurry was kept for 4 hours and the crystals were subsequently collected by filtration. The crystals were washed sequentially with 2:1 DMF:water mixture (2.1 vol), water (2 vol) and 3:1 cold acetonitrile:water mixture (1.5 vol). The wet filter cake was dried at less than 45 °C until the weight loss (LOD) was less than 1%, giving a final yield of about 49%. The product was confirmed by 1H NMR (CDCl3): δ 5.64 (s, 2H), 6.87 (d, 1H, J = 9.4 Hz), 6.95 (m, 2H), 7.30 (m, 1H), 7.42 (d, 1H, J = 9.2 Hz), 8.23 (dd, 1H, J = 10Hz and 2Hz), 8.26 (s, 1H), 8.72 (d , 1H, J = 2Hz). Mass spectrum (MS): 272 (M + H)+; HPLC retention time: 6.99 min (YMC ODS-A 3 µm, 4.6 x 50 mm column, 10 min gradient, 2.5 mL/min).
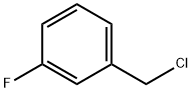
456-42-8
334 suppliers
$5.00/5g

5401-94-5
368 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
50 suppliers
$108.00/1g
202197-31-7
23 suppliers
$463.00/1g
Yield:529508-58-5 33% ,202197-31-7 90%
Reaction Conditions:
with Ki;potassium carbonate in water;N,N-dimethyl-formamide;
Steps:
1.E E.
E. Preparation of 1-(3-Fluoro-benzyl)-1H-indazol-5-ylamine A mixture of 5-nitro-1H-indazole (8.15 gm, 50 mmole), m-fluoro-benzyl chloride (7.95 gm, 1.1 equiv), K2CO3 (7.59 gm, 1.1 equiv), and KI (8.47 gm, 1.02 equiv) in dry DMF (75 mL) was heated at 70° C. overnight. After cooling to RT, water (75 mL) was slowly added to give a precipitate that consisted of about a one to one mixture of isomers [HPLC Ret Time: 1.92 (1-substitued isomer vs. 2.03 (2-substituted isomer) YMC C18 S5 4.6*50 mm, 3 min gradient, 4 mL/min]. This was collected by filtration and washed with water. The solid was crystallized twice from acetone/water to afford the desired 1-(3-fluoro-benzyl)-5-nitro-1H-indazole (4.47 gm, 33%). A suspension of this material (3.00 gm, 11.1) and 10% Pd/C (3.00 gm) in EtOH (21 mL) was kept under an H2 atmosphere (balloon) for 24 hr. The catalyst was removed by filtration and the solvent was evaporated to leave the product as a solid (2.4 gm, 90%). 1H NMR (CDCl3): δ 3.61 (br s, 2H), 5.52 (s, 2H), 6.81-7.85 (m, 7H), 7.85 (s, 1H); MS: 242 (M+H)+; HPLC Ret Time: 1.03 min (YMC Xterra ODS S7, 3.0*50 mm column, 2 min gradient, 5 mL/min).
References:
US2003/186983,2003,A1

456-41-7
296 suppliers
$13.00/25g

5401-94-5
368 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
50 suppliers
$108.00/1g

456-41-7
296 suppliers
$13.00/25g

5401-94-5
368 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
50 suppliers
$108.00/1g
![2H-Indazole, 2-[(3-fluorophenyl)methyl]-5-nitro-](/CAS2/GIF/884874-01-5.gif)
884874-01-5
2 suppliers
inquiry

456-41-7
296 suppliers
$13.00/25g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
50 suppliers
$108.00/1g

456-41-7
296 suppliers
$13.00/25g

5401-94-5
368 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
50 suppliers
$108.00/1g
![1H-Benzimidazole, 2-[(3-fluorophenyl)methyl]-5-nitro-](/CAS/20180601/GIF/833474-48-9.gif)
833474-48-9
0 suppliers
inquiry