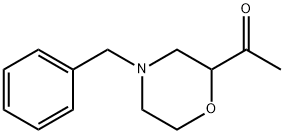
1-(4-Benzylmorpholin-2-Yl)Ethan-1-One synthesis
- Product Name:1-(4-Benzylmorpholin-2-Yl)Ethan-1-One
- CAS Number:852237-34-4
- Molecular formula:C13H17NO2
- Molecular Weight:219.28
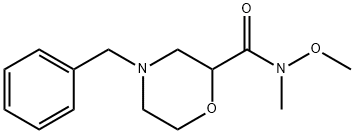
852237-32-2
1 suppliers
inquiry

75-16-1
274 suppliers
$12.00/10ml

852237-34-4
12 suppliers
$45.00/10mg
Yield:852237-34-4 49%
Reaction Conditions:
Stage #1: 4-benzyl-N-methoxy-N-methylmorpholine-2-carboxamide;methylmagnesium bromide in tetrahydrofuran at 0 - 20; for 0.75 - 2 h;
Stage #2: with water in tetrahydrofuran;
Steps:
1
General Synthetic Procedures for the Preparation of Examples 1 to 32 and 35 to 38 General Procedure 1 : Preparation of N-benzyl morpholine alkyl ketones; To a solution of the carboxamide 2 in anhydrous THF at 0°C is added a solution of the requisite Grignard reagent (1.2-3 eq in one or two aliquots). The reaction mixture is allowed to warm up to room temperature and left stirring for 45 minutes to 2 hours before quenching either with 1M hydrochloric acid or saturated ammonium chloride solution and extracting either in DCM or ethyl acetate. The combined organic layers are dried over magnesium sulphate, filtered and concentrated in vacuo to give the corresponding alkyl ketones 3-10,77 and 82.; -4- (Phenylmethyl) morpholin-2-ylJethan-1-one (3; Compound 3 is obtained from 2 (0.730 g, 2.8 mmol) and commercially available (Aldrich) methyl magnesium bromide (1M solution in THF, 3 mL, 3 mmol, 1.1 eq) in anhydrous THF (25 mL) following General Procedure I after purification by automated column chromatography (eluent EtOAc/n-heptane 14/86-100/0 [v/v]) (0.3 g, 49%). MW 235. 33 ; Cl4H2iN02. LCMS (6 minute method) m/z 220.1 [M+H] +, RT 1. 55 min.
References:
WO2005/47272,2005,A1 Location in patent:Page/Page column 45-46; 53
769087-80-1
110 suppliers
$45.00/1g

852237-34-4
12 suppliers
$45.00/10mg