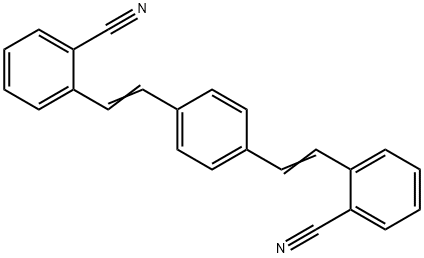
1,4-Bis(2-cyanostyryl)benzene synthesis
- Product Name:1,4-Bis(2-cyanostyryl)benzene
- CAS Number:13001-39-3
- Molecular formula:C24H16N2
- Molecular Weight:332.4
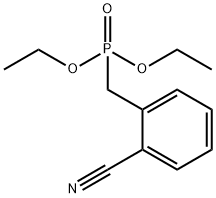
23973-65-1
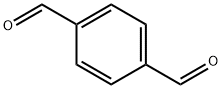
623-27-8

13001-39-3
At atmospheric pressure, 498.6 g of triethyl phosphite and 1000 mL of p-xylene were added to a closed reactor equipped with a reflux condenser and heated to 140°C. Subsequently, 151.6 g of o-cyano-benzyl chloride solution (the molar ratio of triethyl phosphite to o-cyano-benzyl chloride was 3:1) was slowly added, and the addition process lasted for 2 hours. After completion of the reaction, the reaction conditions were maintained for 10 hours. Excess triethyl phosphite and reaction solvent were recovered by distillation for reuse. The reaction mixture was cooled to 25-30°C and dissolved in 500 mL of N,N-dimethylformamide (DMF). Under stirring, 67 g of terephthalaldehyde and 180 g of 30% sodium methanol methanol solution were added to the solution, maintaining an equimolar ratio, and the dissolution process continued for 3 hours. At the end of the reaction, the temperature was lowered to 35 °C and kept for 3 h, then further cooled to 30 °C. After adjusting the pH to 7, the solution was cooled to 10-15 °C and centrifuged to obtain the crude product. The crude product was refined with methanol, centrifuged again and dried to give 1,4-bis(o-cyanostyryl)benzene in 92% yield and 98% purity. Distillation of the mother liquor to remove DMF and methanol (DMF was recovered for the condensation process and methanol was used for the refinement of the crude product) gave diethyl phosphate in 91% yield and 96% purity.
Yield: 91%
Reaction Conditions:
with sodium methylate in methanol;N,N-dimethyl-formamide at 35; for 3 h;Green chemistry;Solvent;Reagent/catalyst;
Steps:
3
Under atmospheric pressure, 498.6 g of triethyl phosphite and 1000 ml of p-xylene were charged into a closed reactor equipped with a reflux condenser, heated to 140 ° C, and 151.6 g of an o-cyanobenzyl chloride solution (The molar ratio of triethyl phosphite and o-cyanobenzyl chloride is 3: 1), the feeding time is 2h, after the reaction is kept for 10h after completion of the reaction, the excess triethyl phosphite and the reaction solvent are recovered by distillation and reused; The resulting reaction mixture was cooled to 25-30 ° C, dissolved in 500 ml of N, N-dimethylformamide (DMF), dissolved in equimolar amounts for 3 hours under stirring, and 67 g of terephthalic aldehyde and 180 g of 30% Sodium methoxide methanol solution, the temperature dropped to 35 ° C after the end of the incubation reaction 3h, then cooled to 30 ° C, adjust the PH value to 7, cooled to 10 ~ 15 ° C, centrifuged, the resulting crude product was refined methanol , Finely centrifuged, and the crude product was centrifuged and dried to obtain 1,4-bis (o-cyanostyryl) benzene. The yield was 92% and the purity was 98%. The mother liquor was distilled to remove DMF and methanol [DMF back to the condensation process, methanol to refined crude], to give two phosphate Ethyl ester, yield 91%, purity 96%.
References:
CN107673998, 2018, A Location in patent:Paragraph 3; 6; 7; 8