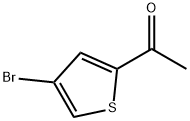
1-(4-BROMO-2-THIENYL)ETHAN-1-ONE synthesis
- Product Name:1-(4-BROMO-2-THIENYL)ETHAN-1-ONE
- CAS Number:7209-11-2
- Molecular formula:C6H5BrOS
- Molecular Weight:205.07
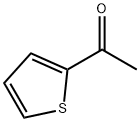
88-15-3

7209-11-2
General procedure for the synthesis of 4-bromo-2-acetylthiophene from 2-acetylthiophene: In a 250 mL single-necked round-bottomed flask, 2-acetylthiophene (3.00 g, 23.81 mmol), anhydrous aluminum chloride (9.53 g, 71.43 mmol), and carbon tetrachloride (80 mL) were added, and the mixture was cooled to -40 °C. After 10 min, a slow dropwise addition of bromine ( 3.81 g, 23.81 mmol) in carbon tetrachloride (20 mL) was added slowly and stirred at room temperature for 0.5 h after completion of the addition. After 12 hours of reaction, TLC monitoring showed complete consumption of the feedstock. The reaction mixture was carefully poured into a mixture of saturated sodium hydroxide solution and crushed ice and extracted with ethyl acetate (75 mL x 3). The organic phases were combined, washed twice with saturated brine and dried over anhydrous magnesium sulfate. Purification by column chromatography (petroleum ether:ethyl acetate = 500:1) gave a pale yellow oily liquid product (2.74 g) in 72.6% yield.

88-15-3
609 suppliers
$5.00/10g

7209-11-2
111 suppliers
$16.00/250mg
Yield:7209-11-2 72.6%
Reaction Conditions:
with aluminum (III) chloride;bromine in tetrachloromethane at -40 - 20; for 12.5 h;
Steps:
1 2- Acetyl-4-bromothiophene (M-1)
In 250ml single neck flask of 2-acetyl-thiophene 3.00g (23.81mmol), anhydrous aluminum chloride 9.53g (71.43mmol) and carbon tetrachloride 80ml, cooled to -40 ° C, was slowly dropped stable after 10min bromo 3.81g (23.81mmol) in carbon tetrachloride (20ml), 0.5h drop was completed, stirring at room temperature. 12After h TLC showed the starting material is no longer remaining. The reaction was poured into a saturated NaOH solution and crushed ice, extracted with ethyl acetate (75ml × 3), the combined organic phase was washed twice with saturated brine, dried over anhydrous MgSO 4. Column layer (PE:EA = 500:1), a pale yellow oily liquid 2.74g, 72.6% yield concentrated under reduced pressure.
References:
China Pharmaceutical University;Lu, shuai;Zhang, Liang;Liu, Hai Chun;Guo, xiaoxing;sun, Shan Liang;Chen, Ya Dong;Lu, tao CN103408540, 2016, B Location in patent:Paragraph 0096; 0126; 0127
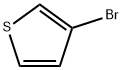
34878-46-1
8 suppliers
inquiry

7209-11-2
111 suppliers
$16.00/250mg
872-31-1
357 suppliers
$9.00/1g
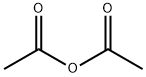
108-24-7
0 suppliers
$14.00/250ML

7209-11-2
111 suppliers
$16.00/250mg

872-31-1
357 suppliers
$9.00/1g

108-24-7
0 suppliers
$14.00/250ML

7209-11-2
111 suppliers
$16.00/250mg
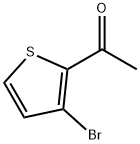
42877-08-7
108 suppliers
$8.00/250mg

872-31-1
357 suppliers
$9.00/1g
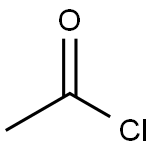
75-36-5
588 suppliers
$17.92/100G

7209-11-2
111 suppliers
$16.00/250mg

42877-08-7
108 suppliers
$8.00/250mg