
1-(4-BROMO-PHENYL)-4-ISOPROPYL-PIPERAZINE synthesis
- Product Name:1-(4-BROMO-PHENYL)-4-ISOPROPYL-PIPERAZINE
- CAS Number:844431-60-3
- Molecular formula:C13H19BrN2
- Molecular Weight:283.21
66698-28-0
151 suppliers
$10.00/250mg

67-64-1
0 suppliers
$19.10/10ml

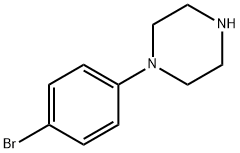
844431-60-3
27 suppliers
$45.00/10mg
Yield:844431-60-3 51 %
Reaction Conditions:
with sodium tris(acetoxy)borohydride in dichloromethane at 20;
Steps:
368.2 Step 2 1-(4-Bromophenyl)-4-isopropylpiperazine
[01001] To a solution of 1-(4-bromophenyl)-piperazine (10 g, 41.4 mmol) and anhydrous acetone (2.4 g, 41.4 mmol) in ethylene dichloride (100 ml_), was added NaBH(OAc)3 (13 g 62.1 mmol) and resultant reaction mass was stirred at room temperature for 1 h. After 1 h, another portion of anhydrous acetone (2.4 g, 41.4 mmol) was added and the reaction was stirred at room temperature for 16 h. After 16 h another portion of acetone (1.2 g, 20.7 mmol) was added and the reaction was further stirred for 6 h. The mixture was treated with 1 N NaOH solution (100 mL) and extracted with diethyl ether (2 x 200 mL). The combined organic layer was washed with brine solution (100 mL), dried over anhydrous Na2S04 and concentrated under vacuum to get crude. The crude was purified by column chromatography and the product was eluted in 4% MeOH in CH2CI2 to afford the product (6 g, 51%) as a yellow solid. LCMS: [M + 2H]+ = 285.27
References:
WO2022/226668,2022,A1 Location in patent:Paragraph 01000-01001