
1-(4-CHLOROPHENYL)-2-METHYLPROPAN-1-AMINE synthesis
- Product Name:1-(4-CHLOROPHENYL)-2-METHYLPROPAN-1-AMINE
- CAS Number:78469-10-0
- Molecular formula:C10H14ClN
- Molecular Weight:183.6779
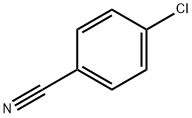
623-03-0
480 suppliers
$6.00/25g
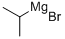
920-39-8
229 suppliers
$85.70/4x25ml

78469-10-0
22 suppliers
inquiry
Yield:78469-10-0 18%
Reaction Conditions:
Stage #1: 4-Cyanochlorobenzene;isopropylmagnesium bromide in tetrahydrofuran at 20;Inert atmosphere;
Stage #2: with sodium tetrahydroborate in tetrahydrofuran;methanol; for 0.5 h;
Steps:
40.a (a) 1-(4-chlorophenyl)-2-methylpropan-1-amine:
i-PrMgBr (2N, 3 mL, 6 mmol) was added drop-wise to a solution of 4-chlorobenzonitrile (680 mg, 5 mmol) in THF (dry, 5 mL) at rt under argon atmosphere. After the addition, the mixture was stirred at rt overnight. Methanol (5 mL) was then added, followed by NaBH4 (2.22 mg, 6 mmol). After stirring for additional 30 min, the mixture was quenched with water, and then extracted with EtOAc. The combined organic layers were extracted with HCl (1N). The pH value of aqueous phase was adjusted the pH to 9~10 with NaOH (1N), then extracted with EtOAc again. The combined organic layers were washed with brine, and dried over Na2SO4. After removal of the solvent, the residue was solidified by using HCl/Et2O. The solid was collected by filtration to give the titled compound as a yellow solid (200 mg, yield 18%). LC-MS: m/z = 182, (m+H)+
References:
WO2013/19621,2013,A1 Location in patent:Page/Page column 76