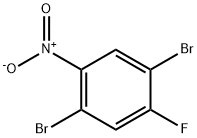
1,4-DibroMo-2-fluoro-5-nitrobenzene synthesis
- Product Name:1,4-DibroMo-2-fluoro-5-nitrobenzene
- CAS Number:366496-33-5
- Molecular formula:C6H2Br2FNO2
- Molecular Weight:298.89
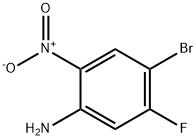
153505-36-3

366496-33-5
General procedure for the synthesis of 1,4-dibromo-2-fluoro-5-nitrobenzene from 4-bromo-5-fluoro-2-nitroaniline: copper(II) bromide (6.37 g, 28.5 mmol) was dissolved in acetonitrile (40 mL) under stirring conditions. N-butyl nitrite (5.0 mL, 42 mmol) was added slowly via syringe and the reaction system was subsequently heated to 60 °C. 4-Bromo-5-fluoro-2-nitroaniline (4.46 g, 19.0 mmol) was dissolved in acetonitrile (60 mL) and slowly added dropwise to the reaction system via a dosing funnel over 15 minutes. The reaction continued to be stirred for 10 min and then cooled to room temperature. The reaction mixture was poured into 2N aqueous hydrochloric acid (400 mL) and extracted with ether. The organic layer was washed with brine and the combined aqueous layers were then extracted with ethyl acetate. All organic layers were combined, dried with anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure by rotary evaporator. Finally, the target product 1,4-dibromo-2-fluoro-5-nitrobenzene was purified by fast column chromatography to give 4.46 g in 76% yield.1H NMR (300 MHz, DMSO-d6): δ 8.59 (d, 1H, J = 6.5 Hz), 8.17 (d, 1H, J = 8.1 Hz).
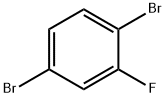
1435-52-5
259 suppliers
$7.00/10g

366496-33-5
59 suppliers
$5.00/250mg
Yield:366496-33-5 89%
Reaction Conditions:
with ammonium nitrate;trifluoroacetic acid;trifluoroacetic anhydride in dichloromethane at 0 - 20;
References:
Chen;Konstantinov;Chittim;Joyce;Bols;Bunce [Environmental Science and Technology,2001,vol. 35,# 18,p. 3749 - 3756]

153505-36-3
75 suppliers
$48.00/500mg

366496-33-5
59 suppliers
$5.00/250mg