1-(4-FLUOROPHENYL)-2-(2-FLUORO-4-PYRIDYL)ETHANONE synthesis
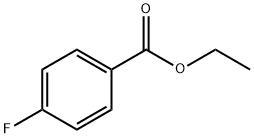
- Product Name:1-(4-FLUOROPHENYL)-2-(2-FLUORO-4-PYRIDYL)ETHANONE
- CAS Number:302839-09-4
- Molecular formula:C13H9F2NO
- Molecular Weight:233.21
Yield:302839-09-4 100%
Reaction Conditions:
with sodium hexamethyldisilazane in tetrahydrofuran at 0 - 20; for 3 h;Inert atmosphere;
Steps:
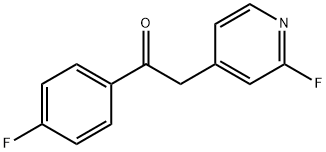
1-(4-Fluorophenyl)-2-(2-fluoropyridin-4-yl)-ethan-1-one (3)
NaHMDS (66.7 mL 2 M solution in THF,133 mmol) was slowly added to a stirred solution of 2-fluoro-4-methylpyridine (10.6 mL, 103 mmol) andethyl 4-fluorobenzoate (18.1 mL, 123 mmol) in 40 mL anhyd. THF at 0 °C under a nitrogen atmosphere.After stirring at 0 °C for 2 h the reaction was allowed to reach rt and stirring continued for 1 h.The mixture was diluted with ethyl acetate and washed twice with 10% aq. HCl. The organic layer wasdried over anhyd. Na2SO4 and solvent was removed under reduced pressure. Recrystallization fromethyl acetate afforded 3 as colorless solid. Yield 23.9 g (quant.); C13H9F2NO (Mr 233.22); m.p. 102 °C;1H-NMR (DMSO-d6): δ= 4.59 (s, 2H, CH2), 7.10 (s, 1H, C3H, Pyr), 7.25-7.27 (m, 1H, C5H, Pyr), 7.37-7.43(m, 2H, C3/5H, F-Phe), 8.11-8.19 (m, 3H, C2/6H, F-Phe, C6H, Pyr) ppm; 13C-NMR (DMSO-d6): δ= 43.6(d, 4JCF = 2.8 Hz, CH2), 110.8 (d, 2JCF = 37.6 Hz, C3H, Pyr), 115.8 (d, 2JCF = 22.0 Hz, C3/5H, F-Phe),123.8 (d, 4JCF = 3.8 Hz, C5H, Pyr), 131.3 (d, 3JCF = 9.6 Hz, C2/6H, F-Phe), 132.9 (d, 4JCF = 2.7 Hz, C1,F-Phe), 147.0 (d, 5JCF = 15.5 Hz, C6H, Pyr), 150.7 (d, 3JCF = 8.5 Hz, C1, Pyr), 163.1 (d, 1JCF = 234.3 Hz,C2F, Pyr), 165.2 (d, 1JCF = 252.0 Hz, C4F, F-Phe), 194.6 (CO) ppm; MS (ESI, 70 eV) m/z 234 [MH]+.
References:
Halekotte, Jakob;Witt, Lydia;Ianes, Chiara;Krüger, Marc;Bührmann, Mike;Rauh, Daniel;Pichlo, Christian;Brunstein, Elena;Luxenburger, Andreas;Baumann, Ulrich;Knippschild, Uwe;Bischof, Joachim;Peifer, Christian;Koch, Pierre;Laufer, Stefan [Molecules,2017,vol. 22,# 4,art. no. 522]
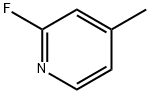
461-87-0
293 suppliers
$10.00/10g
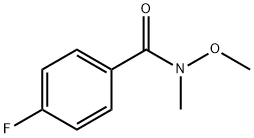
116332-54-8
122 suppliers
$6.00/1g

302839-09-4
12 suppliers
inquiry