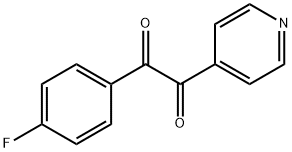
1-(4-Fluorophenyl)-2-(4-pyridinyl)-1,2-ethanedione synthesis
- Product Name:1-(4-Fluorophenyl)-2-(4-pyridinyl)-1,2-ethanedione
- CAS Number:152121-41-0
- Molecular formula:C13H8FNO2
- Molecular Weight:229.21
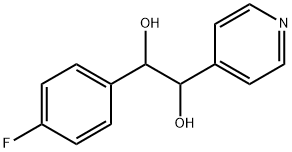
152121-40-9
0 suppliers
inquiry

152121-41-0
26 suppliers
inquiry
Yield:152121-41-0 94%
Reaction Conditions:
Stage #1: 1-(4-fluorophenyl)-2-pyridin-4-ylethane-1,2-diolwith oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -60; for 0.5 h;
Stage #2: with triethylamine in dichloromethane;dimethyl sulfoxide at -60 - 20;
Steps:
13.c
1 -(4-Fluorophenyl)-2-pyridin-4-ylethane-1 ,2-dione To a stirred cooled solution (-6O0C) of oxalyl chloride (3.55 mL, 40.8 mmol) in CH2CI2 (16 mL) a solution of dimethyl sulfoxide (4.0 mL) in CH2CI2 was added dropwise. Five minutes later a solution of 1-(4-fluorophenyl)-2-pyridin-4-ylethane-1 ,2-diol (4.Og, 17 mmols) in DMSO/CH2CI2 (32 mL/13 mL) was dropped. The mixture was allowed to stir for 30 min at -6O0C and then triethylamine (24 mL) was added. When the reaction reached room EPO
References:
WO2007/17096,2007,A1 Location in patent:Page/Page column 58-59
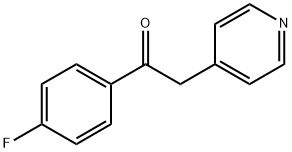
6576-05-2
68 suppliers
$137.00/100mg

152121-41-0
26 suppliers
inquiry

115858-98-5
28 suppliers
inquiry

152121-41-0
26 suppliers
inquiry
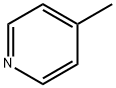
108-89-4
344 suppliers
$14.00/25mL

152121-41-0
26 suppliers
inquiry
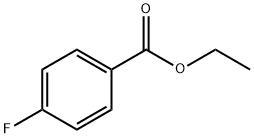
451-46-7
282 suppliers
$5.00/5g

152121-41-0
26 suppliers
inquiry