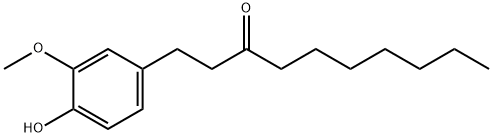
1-(4-hydroxy-3-methoxyphenyl)decan-5-one synthesis
- Product Name:1-(4-hydroxy-3-methoxyphenyl)decan-5-one
- CAS Number:27113-22-0
- Molecular formula:C17H26O3
- Molecular Weight:278.39
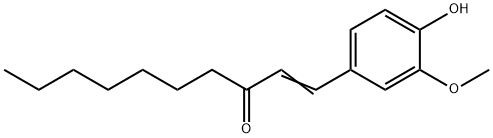
53172-10-4

27113-22-0
Under nitrogen protection, 16 g of sodium metal, 3.5 g of anhydrous sodium acetate and 110 g of tetrahydrofuran were added to the reaction flask and stirred at room temperature. A solution consisting of 50 g of olefinic intermediate and 200 g of ethanol was slowly added dropwise and the dropwise process was completed in about 2 hours. The reaction was then heated and refluxed, and the progress of the reaction was monitored by thin layer chromatography (unfolding agent ratio petroleum ether: dichloroethane = 3:2). After the complete disappearance of the raw material, the reaction was continued for 3.5 hours. The reaction was stopped, 10 g of activated carbon was added and refluxing was continued for 0.5 hours. The reaction mixture was filtered and concentrated under reduced pressure to remove the solvent. The residue was washed with water, layered and dehydrated to give a light yellow oily product. The purity of the product was 98% and the yield was 94%.
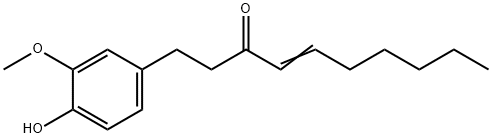
555-66-8
261 suppliers
$35.00/1mg

27113-22-0
218 suppliers
$75.00/250mg
Yield:27113-22-0 98%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in tetrahydrofuran at 20;
Steps:
Synthesis of 1-(4'-hydroxy-3'-methoxyphenyl)-decan-3-one (M11)
Synthesis of 1-(4'-hydroxy-3'-methoxyphenyl)-decan-3-one (M11)
A solution of [6]-shogaol (276 mg, 1.0 mmol) in THF (2 mL) at rt was treated with 10% Pd/C (30 mg, 10% w/w) under H2.
The mixture was stirred at rt overnight and filtered.
The filtrate was concentrated under reduced pressure.
The residue was loaded to preparative TLC (hexane/EtOAc=8:1) to give the title compound M11 as a yellow oil (272 mg, yield 98%); 1H NMR (600 MHz, CDCl3) δ 6.69 (1H, d, J=1.6 Hz, H-2'), 6.82 (1H, d, J=8.0 Hz, H-5'), 6.66 (1H, dd, J=8.0, 1.7 Hz, H-6'), 2.69 (2H, t, J=7.4 Hz, H-1), 2.82 (2H, t, J 7.4 Hz, H-2), 2.37 (1H, t, J=7.4 Hz, H-4), 1.54 (2H, m, H-5), 1.30-1.24 (8H, m, ranged from H-6 to H-9), 0.87 (3H, t, J=6.8 Hz, H-10), and 3.87 (3H, s, OMe-3'); positive APCIMS, m/z 279 [M+H]+.
References:
North Carolina A&T State University;Sang, Shengmin;Chen, Huadong;Zhu, Yingdong US9272994, 2016, B1 Location in patent:Page/Page column 51

53172-10-4
4 suppliers
inquiry

27113-22-0
218 suppliers
$75.00/250mg
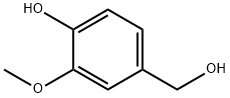
498-00-0
357 suppliers
$21.00/25 g

27113-22-0
218 suppliers
$75.00/250mg
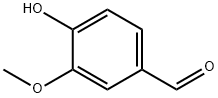
121-33-5
1190 suppliers
$9.00/1g

27113-22-0
218 suppliers
$75.00/250mg
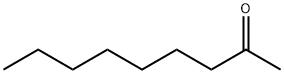
821-55-6
335 suppliers
$7.00/25g

121-33-5
1190 suppliers
$9.00/1g

27113-22-0
218 suppliers
$75.00/250mg