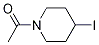
1-(4-Iodo-piperidin-1-yl)-ethanone synthesis
- Product Name:1-(4-Iodo-piperidin-1-yl)-ethanone
- CAS Number:1314310-94-5
- Molecular formula:C7H12INO
- Molecular Weight:253.0807
25503-90-6
294 suppliers
$5.00/1g
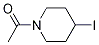
1314310-94-5
3 suppliers
inquiry
Yield:1314310-94-5 87%
Reaction Conditions:
with 1,3-Diiodo-5,5-dimethyl-2,4-imidazolidinedione in 1,2-dichloro-ethane; for 2 h;Product distribution / selectivity;Reflux;Irradiation;
Steps:
3.8
EXAMPLE 3; Radical iodo-de-caboxylation induced by TV-iodo amides/V-iodo amideR-COOH *- R-lGeneral procedure[00111] Procedure: A mixture of R-COOH (1 mmol), N-iodo amide (1-4 equiv), and solvent (3-6 mL) was refluxed (Δ) for 1-24 h in the dark (NL) or under irradiation with 500 W tungsten lamp (TL) or under fluorescent room lighting (FL).[00112] Treatment: The reaction mixture was cooled to rt, and washed with aq NaHS03 and NaHC03 to destroy excess of iodination agent and dissolve unreacted carboxylic acid. The organic solution was dried (Na2S04), filtered through short silica or alumina pad and concentrated in vacuo to give iodide R-I. 100113J Purification: Optionally, the iodide R-I was further purified by crystallization (if the iodide is crystalline compound), or rectification (if the iodide is liquid compound). Analytical sample of the product was purified by column chromatography./V-iodoamideAlk-COOH *- Alk-I[00114] A mixture of Alk-COOH (1 mmol), N-iodo amide (1-3 equiv), and solvent (4 mL) was refluxed (Δ) in the dark (NL) or under irradiation with 500 W tungsten lamp (TL), or under fluorescent room lighting (FL).
References:
WO2011/154953,2011,A1 Location in patent:Page/Page column 28-30