
1,4-Methanonaphthalen-2-amine, 1,2,3,4-tetrahydro- synthesis
- Product Name:1,4-Methanonaphthalen-2-amine, 1,2,3,4-tetrahydro-
- CAS Number:83461-86-3
- Molecular formula:C11H13N
- Molecular Weight:159.23
![Tricyclo[6.2.1.02,7]undeca-2,4,6-triene](/CAS/GIF/4486-29-7.gif)
4486-29-7
2 suppliers
inquiry

83461-86-3
0 suppliers
inquiry
Yield:83461-86-3 98%
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;borane;hydroxylamine-O-sulfonic acid in tetrahydrofuran;
Steps:
2-AMINOBENZONORBORNANE.
2-AMINOBENZONORBORNANE. To a stirred solution of benzonorbornene (2.84 g) in dry THF (8 mL) cooled to 0° C. under an argon atmosphere was added rapidly a 1M solution of borane in THF (6.7 mL). The solution was stirred for 10 minutes at 0° C. then at room temperature for 90 minutes. The reaction mixture was again cooled to 0° C. and hydroxylamine-O-sulfonic acid (1.58 g) was added in one portion. The ice bath was removed and the reaction mixture was stirred at room temperature for 2 hours. 1N HCl(25 mL) and ether (20 mL) were added and stirring continued for 10 minutes. The phases were separated and the organic phase discarded. The aqueous phase was made basic by the careful addition of 50% NaOH solution, then extracted with ether (3*30 mL). The organic phase was dried (MgSO4) and the solvent evaporated to give a yellow liquid (1.35 g) which was 98% pure as judged from GC. The NMR (CDCl3) and GC/MS (m/e=159) were consistent with the title compound.
References:
US6355660,2002,B1
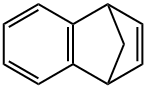
4453-90-1
89 suppliers
$369.00/100mg

83461-86-3
0 suppliers
inquiry
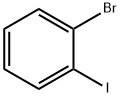
583-55-1
483 suppliers
$7.00/5g

83461-86-3
0 suppliers
inquiry